Phosphorus Analysis. Spectrophotometric Methods
Sample Collection, Treatment, and Stability of the Phosphorus Species. Sampling procedure can be a source of analytical variability, and obtaining a representative and stable sample is paramount in analysis of phosphorus species. Sampling from natural aquatic systems is subjected to seasonal variabilities in phosphorus discharge from anthropogenic sources. The highest phosphorus loadings can correlate with agricultural runoffs during the incidents of increased precipitation, snow melting, or irrigation.
Phosphorus variations can also be caused by phosphorus species leaching from the benthos during anaerobic conditions created in the hypolimnion of a lake. The phosphate is brought to the surface when the surface temperature decreases in the fall and the hypolimnion is mixed with the surface water. By storing surface water samples for four hours at 4 °C, Lambert et al. found no significant change in TP measurements, but observed 25-54% decreases in total dissolved phosphorus, total reactive phosphorus, and dissolved reactive phosphorus. Samples preserved with H2SO4 at pH < 2 can be stored at 4°C for up to 28 days prior to TP analysis. Preliminary treatment is often necessary for the overall effectiveness of the analyses with dependence on the purpose of the phosphorus species determination and complexity of the sample.
Differentiation between dissolved and suspended phosphorus species requires filtration, preferably carried out at the time of the sample collection. Polycarbonate or cellulose acetate membrane filters with 0.45 or 0.2 µm pore size are recommended. However, 0.2-µm-pore-size filter is preferred to remove efficiently bacteria and plankton. Some bacteria, viruses, and colloidal phosphorus can pass through 0.2 pm filter and thus be present in both dissolved and particulate fractions. Further studies on filterability of the freshwater bacteria on 0.1 pm filters were conducted by Wang et al. “Reactive phosphorus" is by definition the phosphorus measured by colorimetric analyses without previous hydrolysis or digestion of the sample, including dissolved and suspended forms of orthophosphate and also condensed phosphates.
The fraction termed “acid-hydrolysable phosphorus" is the portion of organic phosphates converted into orthophosphate by acid hydrolysis at boiling temperatures. “Organically bound phosphorus" is the fraction converted into orthophosphate by a destructive oxidation. Analysis of the TP in water samples requires digestion with oxidizing reagents. The digestion procedures are discussed in Section 2.2.2. However, it is necessary to point out that persulfate digestion produces high concentrations of sulfate, which interferes with ion chromatography (IC) measurements of phosphate, and further sulfate removal techniques are required. Other sample treatment techniques such as removal of interfering matrix anions (off-line or on-line procedures), column-switching systems, and preconcentration are commonly used in chromatographic separations of inorganic noncondensed phosphorus species.
Spectrophotometric Methods. Orthophosphate Analysis. In the early 1900s, Schreiner presented a method of determination of phosphate in waters using ammonium molybdate as a coloring agent. The most common phosphate analysis in water samples is a spectrophotometric (colorimetric) method, a modification of the original Murphy and Riley method using the ascorbic acid as a reducing agent.
Phosphorus analysis by colorimetry involves two general steps: conversion of the phosphorus species into orthophosphate and subsequent colorimetric determination of the orthophosphate. The International Organization for Standardization (ISO) published method 15681 for the determination of orthophosphate by flow injection analysis (FIA) and continuous flow analysis (CFA).
The methods are suitable for the determination of orthophosphate for concentration ranges between 10 and 1000 µg L-1 as P. Samples are mixed with sodium dodecyl sulfate (surfactant) and acidic solution of molybdate and antimony reagents, with molybdenum blue being subsequently produced by the ascorbic acid reduction of a phospho-antimony-molybdate complex and measured at 880nm.
Method ISO 15681 suggests that samples with orthophosphate concentrations lower than 100 µg L-1 as P should be stored in glass containers, while samples with higher concentrations can be also stored in plastic containers. Interferences affecting spectrophotometric determination of the orthophosphate include arsenates (100 µgL-1 as As is equivalent to approx. 30 µg L-1 as P), silicates (at 60 times higher concentrations than P), fluoride (above 50mgL-1), nitrite (above 5 mg L-1 for samples not acidified when collected), and suspended particles blocking the light from the lamp.
Another widely used method for orthophosphate is the vanadomolybdophosphoric acid method. The orthophosphate reacts with ammonium molybdate in the presence of vanadium under acidic conditions and produce a yellow vanadomolybdophosphoric acid, which is measured at 470 nm. The positive interferences for this method are silica and arsenate (when the sample is heated) and negative interferences caused by arsenate, fluoride, thorium, bismuth sulfide, thiosulfate, thiocyanate, and excess molybdate.
Stannous chloride method is based on reduction of molybdophosphoric acid with SnCl2, when an intensely colored molybdenum blue complex is produced and measured at 650 or 690 nm. For samples containing less than 10 µg L-1 as P, the stannous chloride method provides better sensitivity compared to the vanadomolybdophosphoric acid method. Ascorbic acid reduction method is recommended for analyzing samples with orthophosphate concentrations between 10 µg L-1 and 6 mg L-1 as P. Using data provided by Standard Methods, the performance of the three colorimetric methods for measuring orthophosphate is summarized in Figure 2.
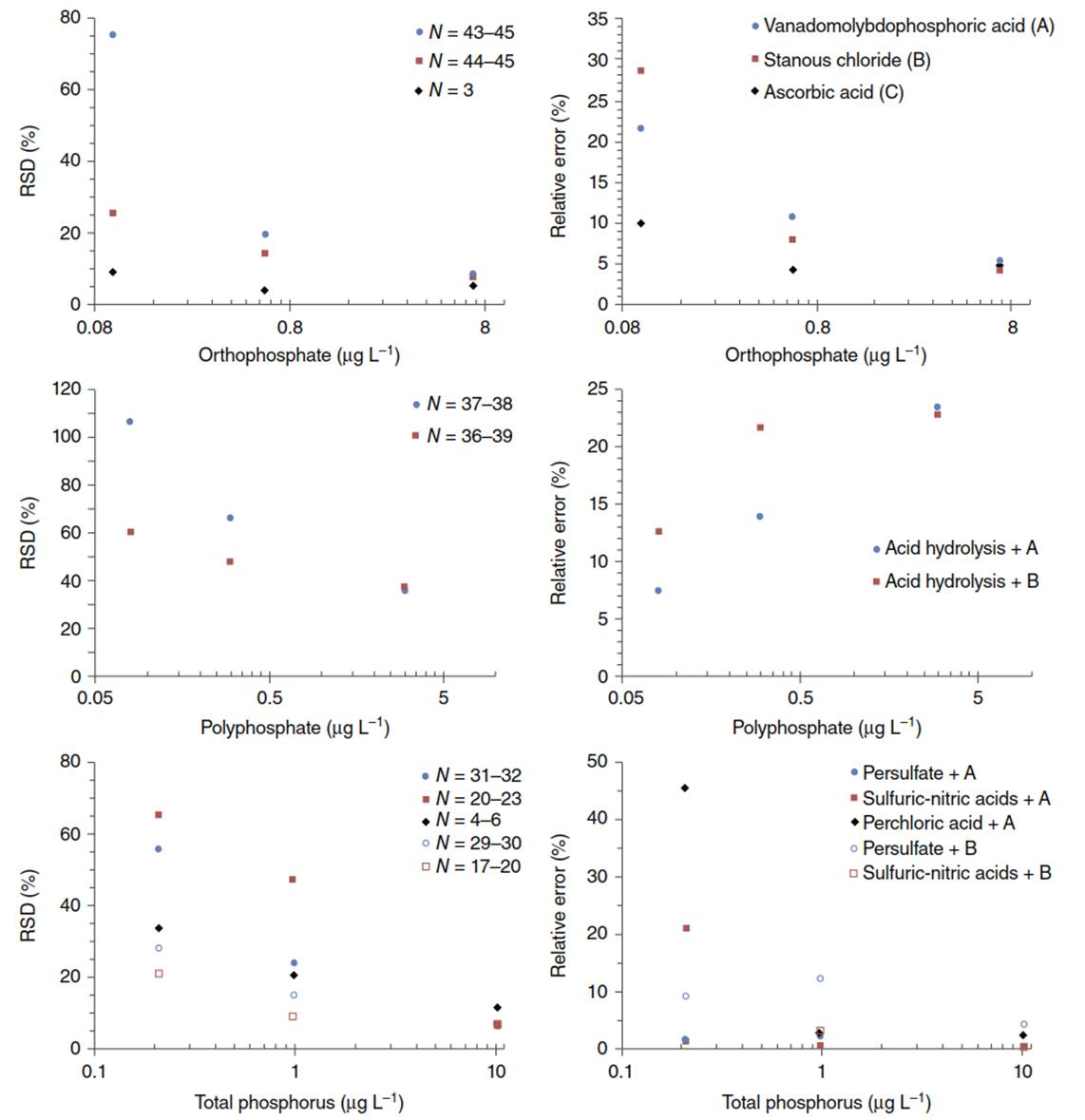
Figure 2. Measurements of orthophosphate, acid-hydrolyzed polyphosphate, and digested total phosphorus using three colorimetric methods (A, B, and C). Comparison of the results is based on the relative standard deviation (RSD) and relative error values provided in table 4500-P:I. of Standard Methods, 22nd edition, where N is the number of participating laboratories (N)
Total Phosphorus Analysis. Digestion of water samples releases phosphorus from all organic phosphorus species and condensed inorganic phosphate. Colorimetric analysis is usually measuring the orthophosphate in the digested samples, being reported as TP. The persulfate digestion by autoclaving at 120 °C was proposed by Menzel and Corwin in 1965, and it is still one of the simplest approaches. In 1974 US Environmental Protection Agency (US EPA) published method 365.4 for TP analysis, with sulfuric acid digestion at 160 and 380 °C in the presence of a mercury catalyst (HgSO4). If this digestion is still in use, the recent implementation of the Minamata Convention on Mercury encourages its replacement with mercury-free alternatives.
The 1978 version of the US EPA method (365.3) introduced the acid persulfate digestion. Standard methods published by the American Public Health Association (APHA) provides digestion procedures with nitric acid-sulfuric acid, perchloric acid, and persulfate, known also as peroxodisulfate. An integrated in-line UV-assisted acid persulfate digestion was also published in ISO method 15681. Alkaline persulfate digestion was proposed by Valderrama [69] in 1981 as a method for simultaneous measurement of TP and total nitrogen. Similar alkaline persulfate digestion methods have been published, with differences in sample volume and in concentrations of persulfate used for digestion (Table 1).
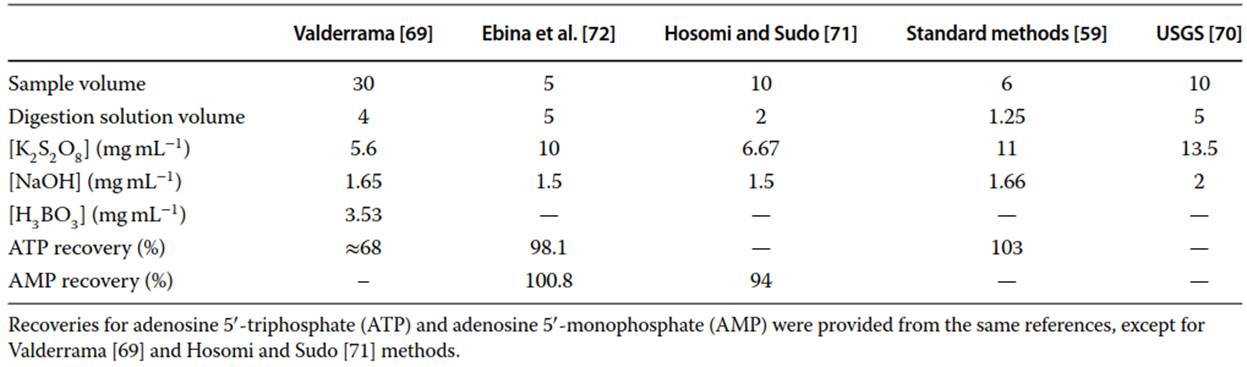
Table 1. Summary of published procedures for alkaline digestions
Ormaza-Gonzalez and Statham concluded that alkaline persulfate digestion method following Valderrama’s procedure has poor recoveries for polyphosphates, metaphosphates, and ATP, while other similar alkaline digestion methods achieve very good recoveries for ATP (Table 1). The UV-based digestion was efficient in releasing phosphorus from sugars, monoesters, and phosphonates, but not efficient for the digestion of polyphosphate, metaphosphate, and ATP.
The results obtained with an in-line automated system with UV-assisted acid persulfate digestion were about 5% biased low compared with manual digestion, mainly due to 90-95% recoveries for pyrophosphate and trimethylphosphate. Due to settling down of the particles in the test tube prior to injection, the method may also not be fit for analyzing samples with high levels of suspended sediments. Lambert and Maher found no significant differences between alkaline persulfate and nitric-sulfuric acid digestion. In a recent study, Dayton et al. confirmed that acid persulfate digestion is much more efficient than alkaline persulfate digestion, particularly for samples containing suspended sediments.
The perchloric acid digestion was more efficient than acid persulfate digestion for water samples with high-suspended sediment levels, but it is labor intensive and requires special safety conditions. Using data provided by Standard Methods, the performance of five different digestion methods is summarized in Figure 2. Arsenate present at higher concentrations than phosphorus and suspended sediments in unfiltered digested solutions can interfere in the colorimetric measurement of orthophosphate. In samples with high levels of iron, phosphorus can be lost by precipitation.
Zhou and Struve found that neutralization and settling time after digestion affect the TP analysis results, especially for samples with very low TP levels even in the absence of visible sediments. For reduced variability in the interlaboratory studies, they also suggested the need for a standardized method providing digestion solution concentrations (H2SO4 and K2S2O8) and detailed treatment procedures following the sample digestion until colorimetric analysis.
Date added: 2025-01-04; views: 727;
