Phosphorus in the Aquatic Environment
Phosphorus in natural waters is present in a variety of chemical forms that migrate between particulate and dissolved fractions, according to a dynamic equilibrium. Orthophosphate (H2PO4- and HPO42- at normal aquatic pH) is a dominant form of dissolved inorganic phosphorus in natural waters with concentrations often at 1 µgL-1 as P or below, usually being below detection limits of the most current methods of analysis.
An extensive steady-state phosphorus radiobioassay study of 56 lakes in North America confirmed that orthophosphate was often present in picomolar (pM) concentrations and was orders of magnitude lower than what can be measured by widely used analytical methods. Other naturally occurring forms of inorganic phosphorus include condensed phosphates such as polyphosphates (chain phosphates), metaphosphates (cyclic phosphates), and ultraphosphates (branched cyclic structures).
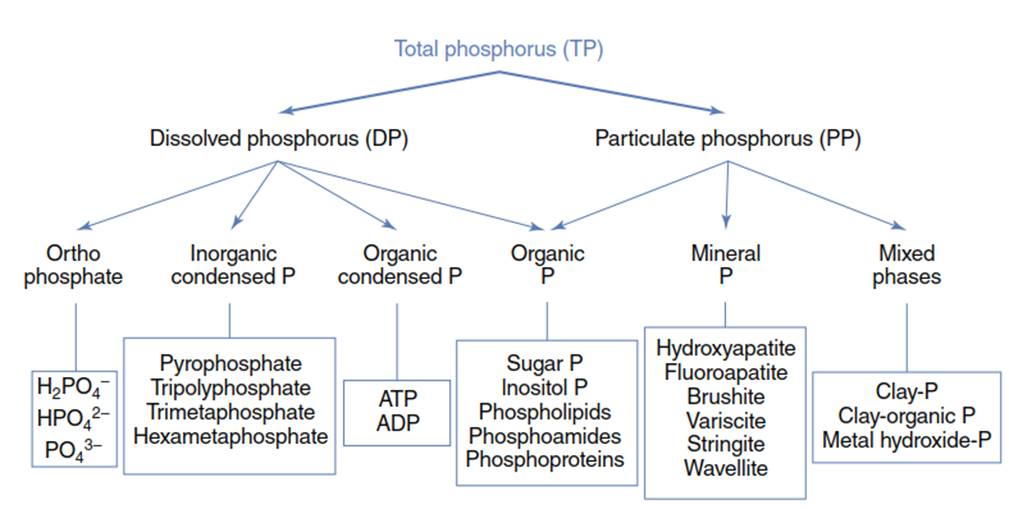
Figure 1. Diagram of P species in natural waters. Source: Adapted from Maher and Woo
Polyphosphates are bioavailable and are produced by a wide range of microorganisms and also by human activities. Pyrophosphate and other polyphosphates usually escape detection by standard colorimetric analytical methods but can be quantified by 31P nuclear magnetic resonance spectroscopy and enzymatic essay studies.
Polyphosphate chainlength effect on phosphatase-mediated hydrolysis mechanisms was investigated with the use of 31P NMR monitoring the dynamic changes of P molecular configuration during polyphosphate hydrolysis. Determination of linear and cyclic polyphosphates by chromatography and electrophoresis methodologies were reviewed by Rosset et al.. Inorganic particulate phosphorus can also release orthophosphate in certain conditions. Iron(III) phosphate or Fe(III) complexes have a major role in cycling the phosphorus within the aquatic environment. Hydrated ferric oxides tend to adsorb dissolved phosphates from the water column and coprecipitate in sediments. During anaerobic conditions, due to iron reduction, phosphorus can be released from the hydrated ferric oxides from sediments into the water column.
Absorption of phosphorus on humic-Fe compounds were studied by Francko and Heath. The research suggested that phosphate adsorbed to oxyhydroxide colloids or ferric-organic colloids can be released and made available to planktonic organisms by photoreduction of iron in the presence of sunlight or by phosphatase activity independently of light intensity. Further studies regarding the biogeochemical cycling of colloidal ferrous oxides in freshwater was investigated by McKnight and Duren.
Organic phosphorus species occurring in water due to biological processes include sugar phosphates, phosphate metabolites (AMP, ADP, ATP), phytate and its degradation products, inositol phosphates and also anthropogenic organophosphorus species such as alkyl substituted phosphonic acids, alkylphosphates and bisphosphonates used as herbicides, pesticides, and detergents. Organic and inorganic phosphorus species in natural water samples including phosphate monoesters, phosphate diesters, polyphosphates, phosphonates, and myo-inositol hexak-isphosphates were detected by 31P NMR spectroscopy.
Dissolved organic phosphorus species make up to 15-30% of the total phosphorus (TP) in lake waters. During summer periods and high biological activity, these compounds may comprise up to 90% of the total soluble phosphorus. A diagram of the phosphorus species in natural waters is adapted from Maher and Woo and presented in Figure 1.
Phosphorus and Eutrophication. Eutrophication of freshwater causes excessive algal growth, depletion of oxygen in deeper regions of aquatic systems, loss of aquatic life, deterioration of aquatic habitat, and threat of toxin contamination in drinking water supplies caused by algal blooms. Eutrophication of surface waters is usually related to the transfer of exogenous forms of phosphorus into surface waters. Phosphorus is often a limiting factor for algal growth in surface waters because it cannot be replenished from atmospheric sources such as nitrogen and carbon. Studies conducted in the Experimental Lake Area have shown that growth of phytoplankton is proportionate to the concentration of phosphorus in the water column.
Further enrichment bioassay studies of phytoplankton growth and its relationship to phosphorus as a limiting factor in freshwater showed that combined nitrogen and phosphorus enrichment enhanced algal growth more substantially than only addition of phosphorus or nitrogen separately. Phosphorus is an essential element and is crucial to metabolism and energy conversion in animals and plants. Plants control the phosphate concentration in the cell by regulating phosphate metabolism.
The only immediately available phosphorus sources for phytoplankton are inorganic phosphate, some phosphorylated sugars, and phosphonates. Total bioavailable phosphorus is a combination of immediately available phosphorus and phosphorus, which can be utilized using desorption, dissolution, and enzymatic degradation.
Orthophosphate and small organic phosphate esters are readily available for phytoplankton uptake. Concentrations of these compounds in natural waters are usually very low, due to high affinity of phytoplankton to readily available phosphorus sources. Another source of phosphorus for the phytoplankton is phosphorus released from the dead cells. The main forms of such phosphorus are inorganic phosphate, sugar phosphates, glycerophosphate, polynucleotides, and phospholipids.
Date added: 2025-01-04; views: 461;
