Analysis of Phosphorus Species in Water
Phosphorus: Occurrence, Production, and Uses. Phosphorus is an element essential for life. It can exist in three main allotropic forms: white, black, and red. A polyatomic nonmetal phosphorus has a single naturally occurring stable isotope, 31P, and is not found in elemental form in nature due to its high reactivity.
In 1669, searching for the mythical “philosopher’s stone", the German physician Hennig Brandt discovered elemental phosphorus while carrying out alchemical experiments with urine. The presence of phosphorus in plants was detected for the first time by Albino in 1688. Almost a hundred years later, in 1769, phosphorus was recognized as an essential ingredient in bones of animals and humans by Gahn and Scheele. The first detection of phosphorus in a mineral (pyromorphite, a lead phosphate) was made by Gahn in 1779.
The name given to the element was taken from the Greek word phosphorous, or “bearer of light’, from the glow of white phosphorus when exposed to oxygen. Phosphorus is located in the 15th group of the periodic table (new IUPAC classification, also previously known as group V of main elements) and belongs to the nitrogen family of elements, known also as pnictogens (nitrogen, arsenic, antimony, bismuth, and a synthetic element named moscovium). White phosphorus is very poisonous to humans with a lethal oral dose of 1mg kg-1 of body weight and as little as 0.2 mg kg-1 may produce adverse effects.
It is absorbed by the liver, and chronic exposure can cause necrosis of the teeth and jaw bones. A characteristic property of phosphorus is the availability of its unfilled 3d orbitals (1s2, 2s2, 2p6, 3s2, 3p3) to form pπ to dπ bonds with oxygen and nitrogen. Because of its smaller radius, phosphorus forms tetrahedral PO43- anions and chain polyacids Hn+2PnO3n+1 (n = 2 - 17).
Phosphorus is the eleventh most abundant element in the earth’s crust with the concentrations to the extent of 1120ppm (mg kg-1). It exists in the terrestrial minerals as orthophosphate with the exception of Schreibersite (Fe, Ni)3P, which is present in meteoric iron. There are around 200 known crystalline phosphate minerals. The major source of phosphorus in rocks is the apatite mineral family Ca10(PO4)6(OH, F, Cl)2, with common members being fluorapatite Ca5(PO4)3F, chlorapatite Ca5(PO4)3Cl, and the most important for industrial applications, hydroxyapatite Ca5(PO4)3OH used to produce phosphate fertilizers.
Phosphoric acid can be obtained using the original Brandt’s method, isolation from urine by distillation, process improved by Robert Boyle in 1680, when fine white sand was added to the mixture to remove volatiles. Later, when the bone ash was used as a raw phosphorus material, sulfuric acid was added to produce phosphoric acid, which was first concentrated and then heated with coke to produce phosphorus. In 1890, Readman improved the efficiency of the process by using an electric furnace based on the following process:
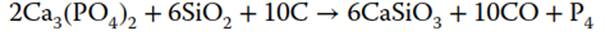
Most of the industrially produced phosphorus is converted into phosphoric acids and their salts. Phosphoric acid is used in production of superphosphates (phosphorus fertilizers for crops) from phosphate minerals and alternate phosphate sources like guano deposits.
Date added: 2025-01-04; views: 495;
