Arsenic in Water: Speciation, Sources, Distribution, and Toxicology
Introduction. Arsenic (chemical symbol: As), a tasteless and odorless tin-white element, has several uses ranging from being part of anticancer drugs to dopants used in semiconductor industries. Table 1 summarizes the physical and chemical properties of the element As. Over time, concerns regarding its toxic impact, on both nature in general, and human beings in particular, has resulted in substantial interest in the sources and mobility pathways of As in environment. A wide variety of studies have focused on mitigating the deleterious impact of As. In parallel, there are ongoing efforts to beneficiate this element.
The World Health Organization (WHO) has declared As-contaminated drinking water as a public health emergency in Bangladesh where thousands of wells containing As have resulted into at least 100 000 cases of skin lesions and other major health issues. In order to continue development of As remediation technologies, it is important for the scientific community to have a deeper understanding of the problem in terms of its emergence, distribution, impact, and existing methods of analysis and treatment. The principal focus of this article is on As treatment and detection in water along with it’s geological, environmental, toxicological, and chemical aspects with a recognition of open challenges that exist in this technology space.
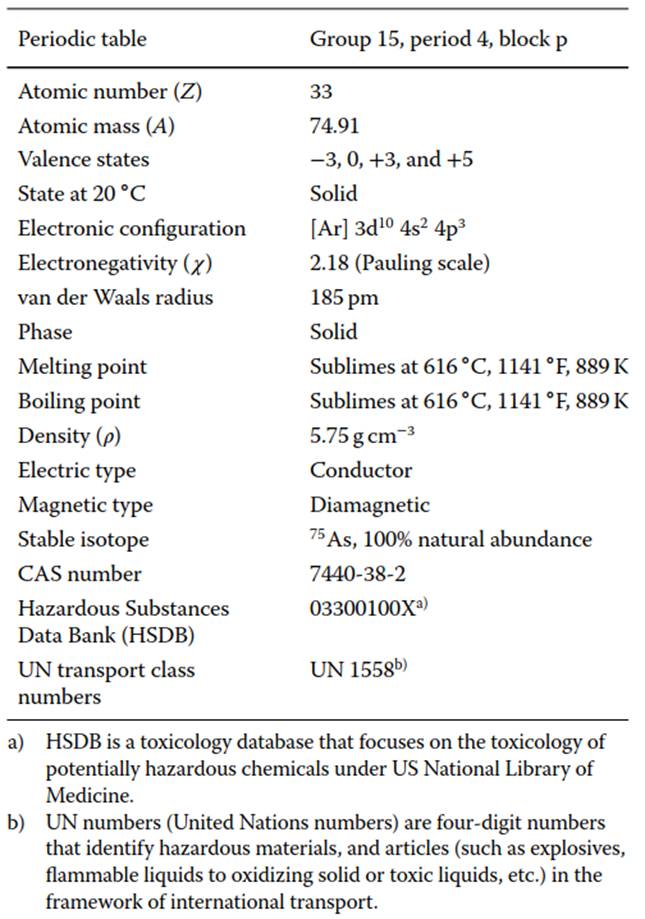
Table 1. As factsheet
Arsenochemistry. As is a metalloid belonging to Group VA of the periodic table. Various species of As are arsenic trioxide (AsO3), arsenous acid (arsenite or As(III)), arsenic acid (arsenate or As(V)), methylated species, arsenobetaine (AB), and arsenocholine (AC). AsO3 has been reported to exist in three allotropic structures. The cubic form, arsenolite is slightly soluble in water, while at higher temperatures, the monoclinic form known as amorphous/glassy claudetite exists. The solubility of arsenic trioxide in 100 mL of water is 1.2 g at 0 °C, 2.1 g at 25 °C, and 5.6 g at 75 °C.
Arsenous acid is an inorganic compound consisting of three hydroxyl groups attached to As in a pyramidal geometry. It occurs in aqueous solutions and may exist as following species H3AsO3, H2AsO3-, or HAsO32-. The hydrolysis of As4O6 gives o-arsenous acid and m-arsenous acid. Owing to similarity in phosphorus and arsenic chemistry, the meta-arsenous acid would be expected to be polymeric like metaphosphate. However, the arsenic-oxygen-arsenic bond is known to possess extreme hydrolytic instability, thereby making the monomeric ortho form as the expected predominant species. Arsenic acid has a tetrahedral molecular geometry and may exist as H3AsO4, H2AsO4-, or HAsO42-. Arsenate is a weak triprotic acid, and arsenite is a hydroxo-acid. The pKa values of arsenate species are:
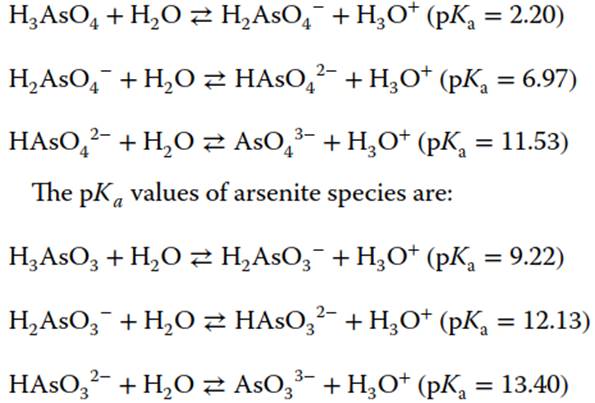
The alkali-metal arsenites are freely soluble in water, the alkaline-earth arsenites are slightly soluble, and the heavy-metal arsenites are insoluble. Methylated organic species of As include dimethylarsinate (DMA) and monomethylarsonate (MMA). Organoarsenic compounds like AB (chemical formula: C5H11AsO2) and AC (chemical formula: C5H14AsO+) are found widely in marine environment and are relatively harmless. Microorganisms play a key role in the As geocycle through the processes of oxidation, reduction, methylation, and demethylation of As species.
Organoarsenic compounds of As(III) and As(V) are also produced by microbiological processes in sediments and soils. Primarily the organoarsenic compounds formed include MMA and DMA. In addition, trimethylarsine oxide (AsO(CH3)3, TMA), phenylarsonic acid (H2AsO3-C6H5, PAA), and some other related species are formed too. Some of this As is liberated into the atmosphere; while some other gets mineralized and gets deposited in the form of sediments.
As may exist in various oxidation states such as (As0), arsenate (As5+), arsenite (As3+), or arsine (As3-). It is generally considered that trivalent As is considerably more toxic than pentavalent As; so it is important to understand whether As in aqueous media exists in the form of arsenite or arsenate - i.e. AsO33- or AsO43-. Lower oxidation state species (0 and -3) occur in strongly reducing conditions, whereas species of higher oxidation states (+5 and +3) occur in oxygenated and mildly reducing conditions, respectively. The mobility of As depends on redox potential (Eh), pH, biological activity, and adsorption/desorption reactions.
Figure 1 is the Pourbaix diagram (Eh-pH diagram) with characteristic Raman spectrum for aqueous As species. Eh is the redox potential of a solution that indicates the activity of electrons in millivolts. pH is the measure of hydrogen ion concentration and thus it indicates the activity of hydrogen ions in a solution. The area on Eh-pH diagram where a mineral or chemical species is stable gives its stability field in that particular range of Eh and pH.
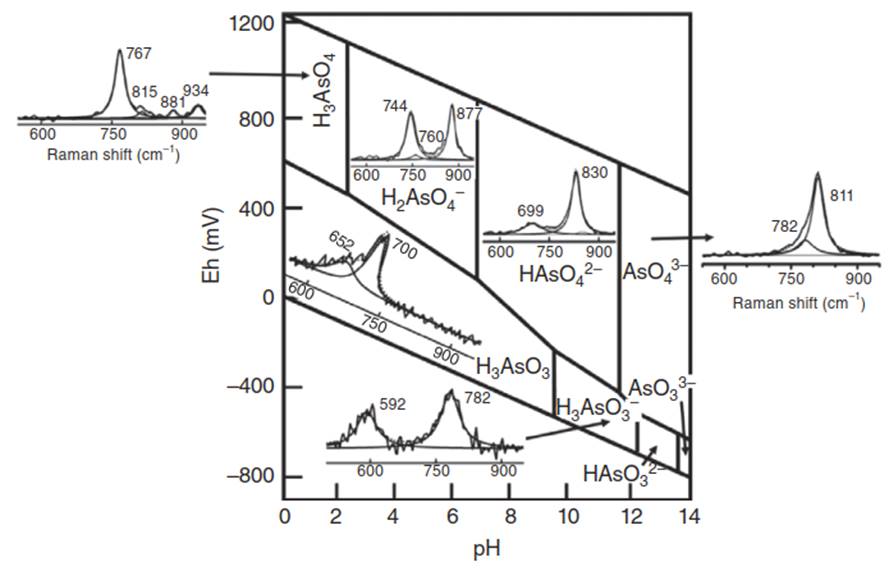
Figure 1. Pourbaix diagram (Eh-pH diagram) with characteristic Raman spectrum for the corresponding aqueous As species. Source: Adapted from Ref. [4, p. 521]. Raman spectrum is one of the ways to identify the As species. These spectra are from our studies
In oxidizing and strongly acidic conditions (pH 2), arsenate exists as H3AsO4. At lower Eh values (in the pH range of 2-10), it dissociates to H2AsO4-, HAsO42- and in strongly basic pH, AsO43- species dominate. Arsenite exists as H3AsO3, in reducing conditions up to a pH of 9. Above this pH, it occurs as H2AsO3-, HAsO32-, and AsO33-. These species can be distinguished by their characteristic Raman features observed at different pH.
From Pourbaix diagram, it can be observed that As species exists in anionic forms in aqueous conditions. These exhibit ligand-like behavior. Arsenite and arsenate show different reactivity with organic sulfur, and nitrogen. Arsenite binds to sulfur and sulfhydryl-containing groups but not to organic compounds containing reduced nitrogen (e.g. amines). Whereas, arsenate reacts with reduced nitrogen groups but not sulfhydryl groups. Presence of sulfide and acidic pH can lead to the formation of arsenic sulfide, HAsS2, and other analogs.
Because of the low solubility of these arsenic sulfides under conditions prevalent in anaerobic aqueous and sedimentary media, these compounds may accumulate as precipitates on minerals like pyrite, realgar, etc. and thus remove As from the aqueous environment. Under strongly reducing conditions, arsenic metal, arsine, and arsine derivatives may exist. As(III) and As(V) are the common species found in contaminated soil and solid wastes, with As(V) as the predominantly adsorbed species on surface of wastes.
Date added: 2025-01-04; views: 662;
