Mass Transfer Effects of Ultrasound
When no NAPL is present, contaminant sorption is quantified by equilibrium soil/sediment-water partitioning coefficient (Kd). This coefficient describes a contaminant’s partitioning to the soil/sediment and aqueous phase. Contaminant association with the soil/sediment phase is described by several inter- molecular forces. Both contaminant and soil/sediment properties influence the attractive forces that control the extent of contaminant sorption.
Most intermolecular forces are driven by enthalpy (∆H). In these cases, interactions between the contaminant and soil/sediment are stronger than interactions between the contaminant and water, and sorption is an exothermic process (∆H < 0), releasing heat. Intermolecular forces that are enthalpically driven include van der Waals forces including London dispersion (induced-dipole-induced dipole), Keesom (dipole-dipole), and Debye (dipole-induced-dipole) forces; hydrogen bonding; electrostatic interactions (ion-ion, ion-dipole); and chemisorption (covalent bonding). Most of these forces are physical adsorption processes, where the contaminant retains its chemical structure, and the interaction forces are weak (low magnitude ∆H).
Chemisorption, another type of sorption, involves the formation of new chemical covalent bonds between the contaminant and soil/sediment. Chemisorption requires high activation energies and often occurs at high temperatures. Therefore, these bonds are stronger and have higher magnitude ∆H. Finally, some sorption processes such as hydrophobic bonding are driven by entropy. Very hydrophobic compounds surrounded by polar water molecules are entropically and thermodynamically unfavorable. Entropy increases when hydrophobic compounds undergo adsorption, making hydrophobic bonding thermodynamically favorable.
Contaminant mass transfer in a soil-sediment/water environment occurs slowly via diffusion. Aqueous phase contamination diffuses from areas of high concentration to areas of low concentration. Contaminants subsequently desorb from the soil/sediment to re-establish equilibrium as described by the Kd partitioning coefficient.
The presence of ultrasound accelerates contaminant mass transfer. Since sorption is an exothermic process, the presence of ultrasound provides energy to shift the contaminant equilibrium toward aqueous phase, rather than the sorbed phase (Le Chatelier’s principle). This phenomenon can be attributed to several physical ultrasonic effects. Localized, turbulent fluid flow from microstreaming enhances the flow of aqueous contaminants away from soils/sediments, encouraging contaminant desorption. Microstreaming has also been ascribed to enhance mass transfer coefficients across solid-liquid interfacial films by flushing contaminants from particle surfaces.
Microscopy images of ultrasonically cleaned membranes corroborate microstreaming as a mass transport mechanism, revealing circular patches on the surface. Interparticle collisions from shockwaves and microjets reduce particle size, as seen in Figure 5. Particle size reduction increases interfacial surface area of sediment and soil particles, and decreases the path length for contaminant desorption. Lastly, large-scale fluid flow from acoustic streaming enhances convective transport of contaminants once in the aqueous phase. By promoting contaminant mass transfer in solid-liquid media, ultrasound reduces the rate-limiting step to remediation of high sorbed contamination.
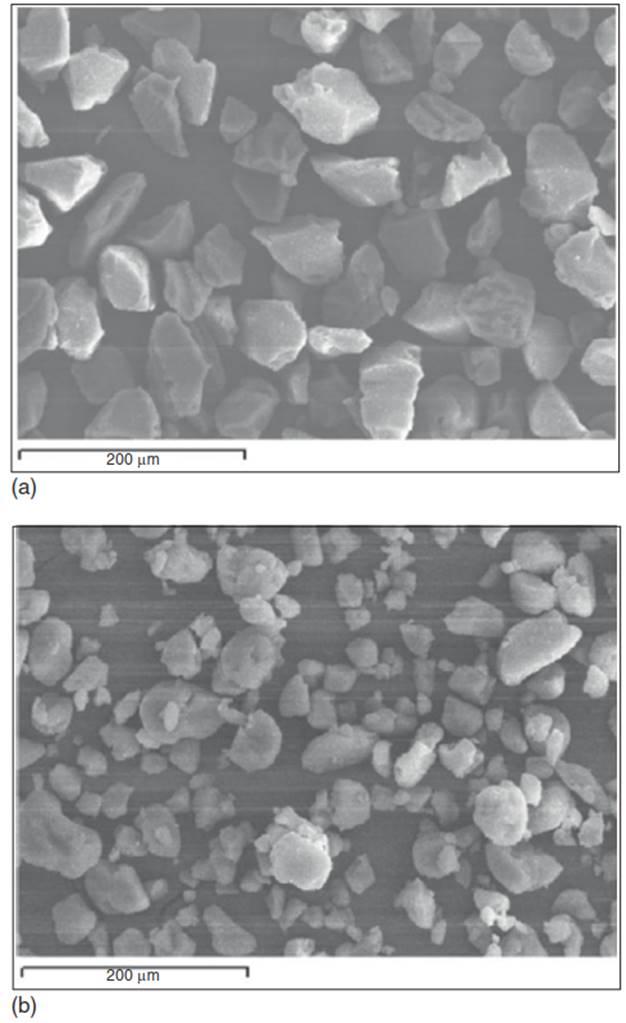
Figure 5. Scanning electron micrograph, SEM, images of silica particles before (a) and after (b) 60 min of sonication
Ultrasound has been shown to enhance both adsorption and desorption of phenols to activated carbon. Schueller and Yang observed a nearly 10-fold increase in desorption rate of phenol in the presence of ultrasound compared to mechanical mixing; parameters such as power input altered the rates and extent of desorption. Moreover, they determined that adsorption was not substantially increased compared to mixing, and attributed it to essentially no activation energy for adsorption, whereas an activation barrier exists for desorption.
Similar to Schueller and Yang, Hamdaoui et al. observed a significant increase in desorption of 4-chlorophenol from activated carbon, but unlike Schueller and Yang they observed a significant increase in adsorption due to ultrasound. Parameters such as power and temperature played a role in rates and extent of adsorption and desorption. In both the cases the total fraction desorbed from activated carbon was a very small fraction of the total contaminant present due to equilibrium conditions with the solution.
This result indicates that while ultrasound does not effectively remove contaminants from activated carbon, ultrasound does desorb contaminants from solids much more than vigorous stirring, suggesting that mass transfer limitations are significantly reduced with the use of ultrasound. Overall, mass transfer from soils/sediments to the aqueous phase by ultrasound improves accessibility for treatment of strongly sorbed contaminants.
Date added: 2025-01-04; views: 372;
