Ultrasound Combined with Other Technologies
Enhancement of fluid flow and solute transport by ultrasound allows for potential coupling to other remediation technologies for in situ treatment of contaminated soils and sediments. Conceivable technologies to combine with ultrasound include amendments (e.g. chemical oxidants and sorbent materials) and biological treatment. Increasing fluid flux is vital for successful amendment delivery to the contaminated source. Combinative treatment may result in synergistic contaminant degradation, and improve the feasibility of ultrasonic applications.
In Situ Chemical Oxidation. In situ chemical oxidation (ISCO) is the subsurface use of highly oxidizing compounds for chemical transformation of harmful organic contaminants, ideally, to less-harmful species. Common oxidants include hydrogen peroxide, ozone, permanganate, and persulfate. Some oxidants require activators to generate highly reactive radical species. Oxidants are characterized by their standard reduction potential, E°, as well as reactivity and persistence in the subsurface. A compound is oxidized when it transfers/loses electrons to an oxidant, or oxidizing agent. Target compounds compete with nontarget compounds, such as organic matter and reduced chemical species, for oxidation by nonselective oxidants.
Oxidant delivery to targeted contamination is a challenge in low-permeability soils/sediments, which have high dispersivity from preferential flow paths. Also, for oxidants with low subsurface persistence, delivery is hindered due to fast reaction rates. Moreover, oxidant and soil mixing are often overestimated, leading to uncertainties in treatment efficacy.
Ultrasound has potential to overcome ISCO limitations by enhancing fluid flow and solute transport, leading to enhanced oxidant delivery to source contamination. Physical ultrasonic effects assist delivery through turbulent mixing and mass transfer of entrapped contaminants. Ultrasound also provides another source of radical species to assist in oxidation.
Persulfate. A common ISCO oxidant is persulfate, S2O82-. Persulfate (E° = 2.1V) is activated to highly reactive sulfate radicals, SO4- (E° = 2.6 V). Typical activation methods include heat (Eq. 4), UV light (Eq. 4), base (Eq. 5), and transition metal (Eq. 6) activation
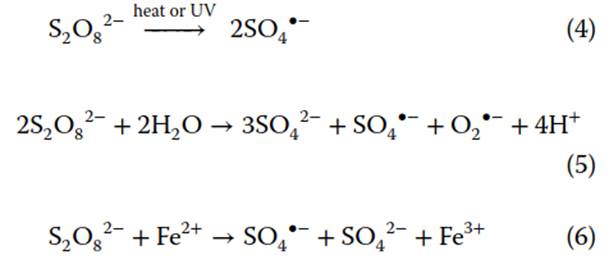
SO4-, while offering higher degradation rate constants than S2O82-, has a short lifetime in the subsurface (seconds). Its short lifetime limits the diffusion distance and oxidant delivery of SO4-. Despite this, SO4- has a longer lifetime than •OH because •OH has faster reaction kinetics due to its relative nonselectivity. SO4- promotes chain-propagating reactions, or reactions that produce additional radical species. For instance, SO4- can react with organic compounds (R) to generate organic radicals (R) (Eq. 7) or at elevated temperature react with water to form •OH (Eq. 8). Radical species generated from chain-propagating reactions also contribute to degradation of target compounds
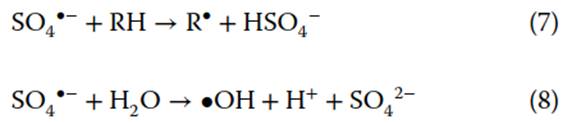
Ultrasound activates persulfate as well. Ultrasonic activation of persulfate occurs at the bubble-water interface, where cavitational heating decomposes persulfate by cleaving the peroxide (R1-O-O -R2) bond. Using electron paramagnetic resonance and spin trapping, a high •OH yield from ultrasonically activated persulfate is ascribed to the hydrolysis of SO4- (equation 8) at the bubble-water interface. Some studies have observed faster degradation of organic contaminants via ultrasonically activated persulfate compared to either persulfate or ultrasound alone. Applications in heterogeneous systems have demonstrated oxidation of soil-sorbed phenanthrene and coal-tar DNAPLs.
Fenton's Reagent. Another common ISCO technology is Fenton’s reagent, sometimes called catalyzed hydrogen peroxide. Hydrogen peroxide, H2O2, is catalyzed by metal cations, such as Fe2+, to form reactive species such as •OH, O2-, and HO2 (Eq. 9). Fe3+ produced is cycled back to Fe2+ through reaction with H2O2 or HO2 (Eqs. 10 and 11). Catalysts may be naturally occurring. Of all reactive species generated, •OH has the largest standard reduction potential (E° = 2.8 V) and highest reactivity, thus, is most effective at degrading organic contaminants
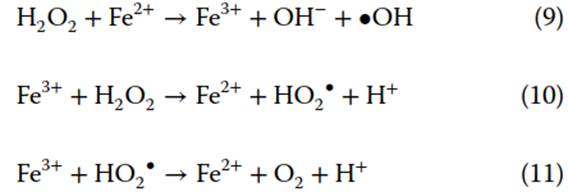
Fenton’s reagent is affected by catalyst solubility. pH
has a strong influence on catalyst solubility and Fenton reagent effectiveness. For instance, Fe cations are more soluble at acidic pH values and are available for catalysis. Highly buffered sediments at pH values unsuitable for catalyst solubility may hinder treatment. Feasibility of pH adjustment may change from site to site. Additionally, the Fenton reaction enhances mass transfer from soils/sediments to the aqueous phase by breaking down organic matter, or enhancing NAPL dissolution from Fenton thermal effects.
The combination of Fenton’s reagent and ultrasound is called the sono-Fenton process. The sono-Fenton process has shown enhanced desorption of naphthalene, toluene, and xylene contaminants from soils. In the presence of a metal catalyst, contaminant oxidation is enhanced from two sources of •OH: Fenton’s reagent and acoustic cavitation. In the treatment of petroleum sludge waste, the sono-Fenton process is more effective in removing contaminants than individual oxidation technologies.
Zero-Valent Iron. Both persulfate and Fenton’s reagent oxidation technologies employ metal cations to activate radical species (Eqs. 6 and 9). An alternative activator that can benefit both treatment technologies is zero-valent iron (Fe0), or ZVI. In the presence of water, ZVI is oxidized to Fe2+ (Eq. 12), which can be used by persulfate and/or hydrogen peroxide to activate radical species. ZVI is unique in that it can react with Fe3+, postactivation, to regenerate Fe2+ (Eq. 13) for further activation of •OH.
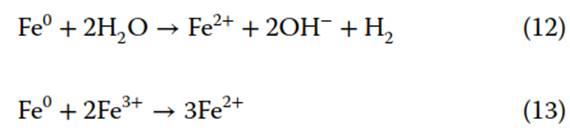
Through exposure to water and oxygen, ZVI can develop a passivating layer of iron oxides, impairing ZVI surface reactivity. The addition of ultrasound can clean and refresh the ZVI surface and accelerate the regeneration of Fe2+. A combined ultrasound/ZVI/persulfate system has been demonstrated to have synergistic removal of pharmaceutical compounds in wastewater. Ultrasound plays a major role in this system by quickening Fe2+ generation by cleaning the ZVI surface and promoting radical reactions in solution. In combined ultrasound/ZVI/Fenton treatment of 1,4-dioxane, ZVI enhanced physical ultrasonic effects through heterogeneous bubble collapse. Additionally, erosion of the ZVI surface exposed more active sites for 1,4-dioxane degradation. While these combined treatment technologies are effective in degrading aqueous contamination, little work has explored the implications on contaminated soils and sediments.
Sorbent Amendments. Instead of using ultrasound to enhance contaminant degradation, ultrasound can be coupled with sorbent amendments to reduce contaminant bioaccessibility. Few studies have considered this combinative technology. Pee studied PAH bioaccessibility in sediments contaminated by a series of PAHs using an activated carbon sorbent in the presence of ultrasound. As shown in Figure 7, PAH contaminants are primarily sorbed to black carbon, soil organic matter, or entrapped in microporous minerals.
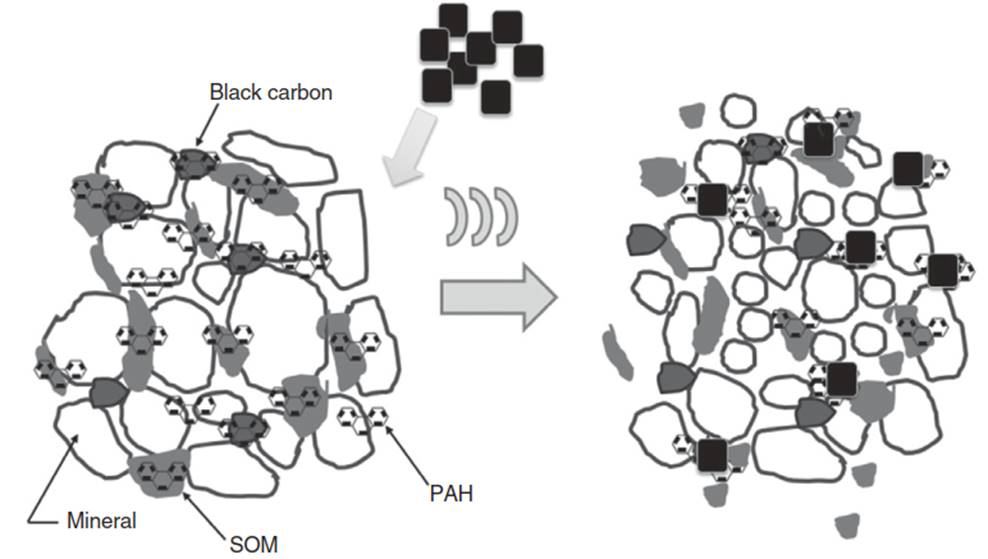
Figure 7. Ultrasonically induced mass transfer of entrapped contaminants from the sediment phase to an activated carbon "catcher.”
Physical ultrasonic effects, such as localized turbulence, microjets, and particle fragmentation, allow for compound desorption into the aqueous phase, followed by enhanced sequestration to the strongly sorbing activated carbon. In other words, contaminants “switch" from sediment to the amendment. This is feasible because ultrasound cannot effectively remove contaminants from strongly sorbing activated carbon. Therefore, sonication is more effective in reducing PAH bioaccessibility compared to mechanical mixing. Although addition of sorbent amendments does not lower contaminant concentrations, ultrasound coupled with sorbent amendments remains a remediation option by accelerating the sorption to activated carbon.
Biodegradation. Biodegradation utilizes microorganisms to degrade contaminants. The extent of biodegradation is dependent on contaminant availability in the aqueous phase. Mass transfer from the sediment to the aqueous phase is limited by small pore sizes, contaminant sorption to organic matter, and slow diffusion. Increasing contaminant desorption in the presence of ultrasound can increase contaminant availability for biodegradation. In addition, ultrasound has been employed as a pretreatment step to aerobic biodegradation of wastewater.
The presence of ultrasound enhances the percent reduction of wastewater substrate. Ultrasound has also been shown to enhance bacteria-mediated hydrolysis of wastewater sludge, increasing hydrolysis products. Moreover, the combination of ultrasound and biomass was shown to treat mercury-contaminated sediments. Ultrasound enhances mercury desorption into the aqueous phase, where algal cells sequester dissolved mercury.
Additional work is encouraged to further mechanistic understanding of different technologies, contaminants, and ultrasonic conditions. Potential synergistic removal using ultrasound coupled with other remediation technologies includes electrokinetics and thermal decomposition. Mechanistic understanding of ultrasound and additional amendments is imperative for effective degradation of target contaminants.
Date added: 2025-01-04; views: 356;
