It Is Not Just the Trajectories: The Tragedy of Repulsion
The other major challenge in prediction is that CFT described earlier fails under environmental conditions because colloids and surfaces in environmental media both tend to be negatively charged. Opposites attract, like-charges repel, and the repulsion calculated for environmental conditions by existing theory is too strong to allow attachment. As a result, theory predicts zero colloid attachment even though we know from experience that attachment occurs; i.e., think about whether you would sooner drink from a pond versus a spring.
Experiments show that under these so-called unfavorable (repulsive) conditions that dominate in the environment, the distribution of colloids with distance from source also becomes unpredictable, showing strange spatial trends that defy existing theory. Some of the observed trends suggest that colloid populations have “stickier" and “less sticky" subgroups wherein the sticky subgroup is retained upstream of the less sticky subgroup.
This situation confounds prediction of the distance, across which, colloids will transport before dropping below a threshold concentration. Hence, until now, we have lacked a theory to guide design of many environmental filtration applications including: (i) novel nanoparticle delivery in the subsurface for subsurface contaminant cleanup; (ii) optimal pumping rates for riverbank filtration as a first step in low-energy water treatment; and (iii) setback distances between drinking water wells and pathogen sources, such as septic systems and livestock operations.
DLVO Theory: Colloid-Collector Interactions. Both favorable and unfavorable colloid-collector interactions are described by DLVO theory, named for the physicists Derjaguin, Landau, Verwey, and Overbeek (DLVO) who developed it. According to classic DLVO theory, the total interaction between a colloid and a surface can be represented by van der Waals and electric double-layer interactions, as well as other interactions described below that are extensions of classic theory. Van der Waals attraction emanates from fleeting and/or permanent dipoles in molecular bonds, is attractive for the vast majority of surfaces, and is considered to be independent of solution chemistry.
The electric double-layer interaction energy is repulsive when the colloid and the grain surfaces are like-charged, and the distance over which this repulsion extends is inversely related to solution ionic strength (IS). Classical DLVO theory states that an increase in IS causes a compression of the electrical double layer surrounding charged particle surfaces. Double-layer compression produces a corresponding reduction in the effective range of repulsive interaction between similarly charged particles.
The profile of net interaction energy (∆G) is shown in Figure 4, where van der Waals attraction greatly dominates at <1nm colloid-collector separation distances corresponding to attachment (primary minimum). Electric double-layer repulsion between like-charged surfaces yields a barrier to attachment at intermediate separation distances, and whose magnitude depends on solution IS. At separation distances greater than the reach of electric double-layer repulsion (e.g. between several tens and ~200 nm), van der Waals attraction dominates, yielding a weakly attractive zone (secondary minimum). The energy barrier to deposition is absent when the colloid and grain surfaces are oppositely charged or when the IS is sufficiently high to compress the electric double-layer repulsion to short separation distances.
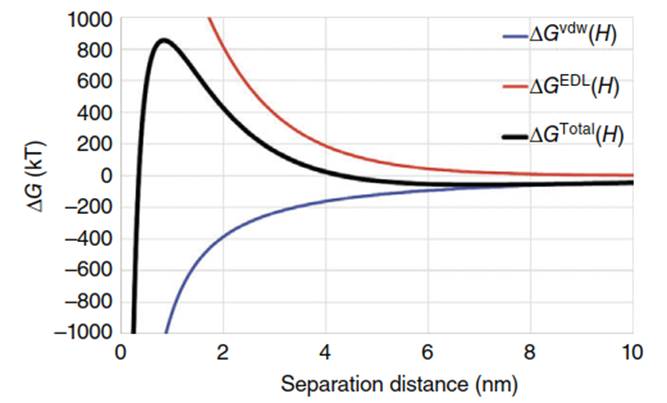
Figure 4. Example colloid-surface interaction (DLVO) profile with van der Waals interaction in blue (bottom curve), electric double-layer interaction in red (top curve), and total interaction in black (intermediate curve). Note that AG is attractive, and positive AG is repulsive
In addition to the traditional DLVO forces described earlier, extended-DLVO forces include short-range interactions such as steric, Lewis acid-base, and Born forces. Repulsive steric forces may arise from interactions of water molecules with surfaces (repulsive hydration forces) and/or from molecular structures on the surfaces. Lewis acid-base forces arise from electron pair donor versus electron pair acceptor interactions and can be attractive or repulsive depending on the relative hydrophilic versus hydrophobic natures of the interacting surfaces. Lewis acid-base forces are significant in systems with strong hydrogen bonding or where other polar interactions are present (common in biological and environmental systems). Born forces arise from strong repulsion due to overlap of electron orbitals of atoms on adjacent surfaces (touching) and only apply at very short distances.
Date added: 2025-02-13; views: 297;
