Components of the Earth System. Atmosphere
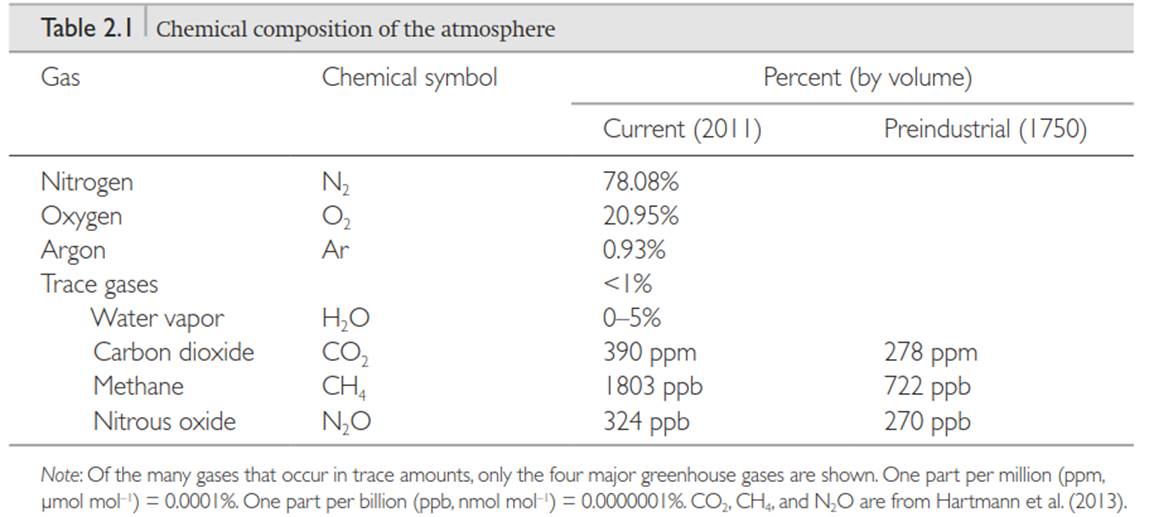
Atmosphere. The atmosphere is the air that surrounds Earth. It is comprised primarily of nitrogen (N2) and oxygen (O2), which together account for 99 percent of the volume of the atmosphere (Table 2.1). Many other gases occur in trace amounts that when combined comprise less than 1 percent of the volume of the atmosphere. Although they occur in minor quantities, some of these gases play an important role in Earth’s radiation balance through the greenhouse effect.
Air pressure is a measure of the mass of air above a given point. The total pressure exerted by a parcel of air is the sum of the pressures of all the individual gases in the parcel. Nitrogen, which comprises 78 percent of the air, exerts the most partial pressure, followed by oxygen (21%). Water vapor typically comprises 1-4 percent of air. For example, the atmospheric pressure near sea level is about 1000 hectopascals (hPa, 1 hPa = 100 Pa = 1 millibar). The partial pressure of nitrogen is 780 hPa and oxygen is 210 hPa. If water vapor comprises 1 percent of the parcel, its partial pressure is 10 hPa or 1000 Pa. Because water vapor is only a small constituent of air, vapor pressure is only a small component of total air pressure. Carbon dioxide has a partial pressure of about 40 Pa.
Greenhouses gases are poor absorbers of solar radiation, but are strong absorbers of longwave radiation. As a result, the Sun’s radiation passes through the atmosphere and heats the surface, but greenhouse gases in the atmosphere absorb the longwave radiation emitted by the surface. The majority of this longwave radiation is emitted back to the surface, warming the surface. This reemission of terrestrial longwave radiation back to the surface is the greenhouse effect that warms the surface.
The principal greenhouse gases are water vapor (H2O), carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O). The amount of water vapor in the atmosphere varies geographically and seasonally and can be as high as 5 percent of the atmosphere, with more water vapor in the warm tropics than in colder polar regions and more water vapor in warm seasons than in cold seasons. The concentrations of CO2, CH4, and N2O vary over time. The concentration of CO2 in the atmosphere averaged for the year 2011 was 390 parts per million (ppm, or pmol mol-1; more precisely, this is the mole fraction, defined as the number of CO2 molecules in a given number of molecules of dry air) - a 40 percent increase from preindustrial levels, primarily as a result of human activities such as fossil fuel burning and deforestation.
The concentrations of CH4 and N2O have similarly increased over the past few centuries. Although these gases occur in lower concentration than CO2, their global warming potential is much greater. Global warming potential measures the effectiveness of gases in absorbing outgoing terrestrial radiation combined with their lifetime in the atmosphere. It is a measure of the total energy added to the climate system relative to that added by CO2. The 100-year global warming potential of CH4 is 28 times that of CO2, and N2O has a 100-year global warming potential 265 times that of CO2 (Myhre et al. 2013). Ozone and halocarbons (chlorofluorocarbons (CFCs), hydrofluorocarbons (HFCs), and other carbon compounds containing fluorine, chlorine, bromine, or iodine) are other greenhouse gases.
In addition to these gases, the atmosphere contains microscopic particles ranging in size from a few nanometers to tens of micrometers, known as aerosols (Boucher et al. 2013). Primary aerosols enter the atmosphere directly as dust from land, salts from ocean spray, black carbon (soot) from fires, and volcanic ash. Wind erosion from arid and semiarid environments carries the largest amount of mineral aerosols into the atmosphere. Sea salt produced by breaking waves is a similarly large source of aerosols. Secondary aerosols form when chemical reactions in the atmosphere convert emitted gases to particles. Biological processes on land and in oceans emit sulfate and organic particles. Over land, organic condensates form during chemical reactions involving the emission of nonmethane hydrocarbons from terrestrial vegetation. Human activities produce a wide variety of primary and secondary aerosols, though the total emission is small compared with natural emissions. Sulfate aerosols produced through the combustion of sulfur-containing fossil fuels are particularly important. Airborne particles such as dust and sulfate aerosols directly affect climate by absorbing or scattering solar radiation. In addition, sulfate aerosols alter climate indirectly by influencing the number and size of cloud droplets. In this way, clouds brighten, reducing the sunlight reaching the surface. Black carbon deposited on snow and ice darkens the surface and enhances solar heating of the surface.
The atmosphere is constantly in motion. These motions arise from the heating of the air, land, and oceans by the Sun and the resultant geographic redistribution of heat by atmospheric and oceanic circulations. These motions can be seen over periods of a few minutes in the fluttering of flags in a strong breeze, over the course of a day in the formation of cumulus clouds and afternoon thunderstorms, or in the passage of warm or cold fronts. Atmospheric phenomena at these short timescales are referred to as weather. Whereas weather can be thought of as describing the instantaneous state of the atmosphere, climate describes the average weather, or long-term state of the atmosphere, over periods of many years.
Earth’s surface has distinct climate zones defined by temperature and precipitation (Figure 2.1). Tropical climates occur near the equator where monthly mean temperatures are warmer than 18°C. Polar climates form near the poles, where the warmest month of the year is colder than 10°C. Polar climates are categorized as tundra or ice cap based on temperature. In between are the middle latitude climates with both warm and cold seasons. Several such mid-latitude climates form depending on temperature. For example, the subarctic climate zone has less than four months with monthly mean temperature greater than 10°C. Similarly, there are major geographic precipitation patterns. Tropical regions along the equator receive abundant rainfall year-round (tropical rainforest climate). Other tropical regions receive less annual rainfall and have pronounced wet and dry seasons (tropical savanna). Middle latitudes are generally moist, though arid climates, categorized as semiarid or arid based on decreasing moisture, develop along the subtropical high pressures at latitude 30° in both hemispheres and in regions far removed from sources of atmospheric moisture. The Mediterranean climate is a distinct mid-latitude climate with a dry season in summer. Polar regions are generally dry because the cold air holds little moisture.
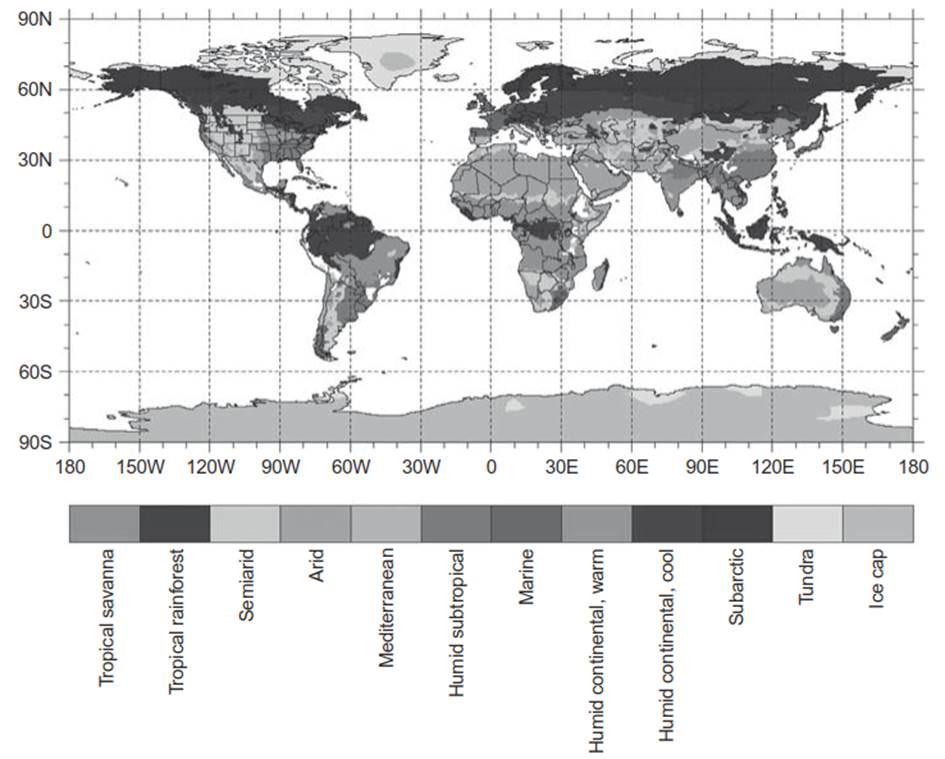
Fig. 2.1. Earth’s major climate zones following the Köppen classification as modified by Trewartha (Chapter 6). See color plate section
Earth’s climate has changed in the past, and is changing still. Global mean planetary temperature increased by 0.85°C over the period 1880-2012 (Hartmann et al. 2013). Human activities that increase greenhouse gases and aerosols in the atmosphere have contributed to this climate change (Bindoff et al. 2013).
Date added: 2025-05-15; views: 253;
