Crystal Form and Crystal Habit
One of the most aesthetically pleasing aspects of mineral specimens in museum exhibits, in mineral shows, or in private collections is their occurrence in well-formed crystals or crystal groupings. Crystals are bounded by smooth plane surfaces and assume regular geometric forms (see Fig. 2.1). The regular geometric shape of a, crystal, its crystal form, is not only pleasing to the eye but is also a diagnostic physical property. Mineral specimens that exhibit good crystal outline have formed under favorable geological conditions and are commonly the result of chemical deposition from a solution (or melt) in an open space, such as a vug or cavity in a rock formation.
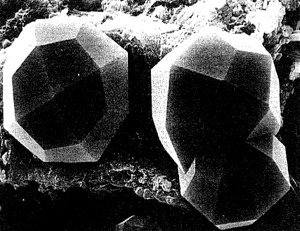
FIG. 2.1. Scanning electron microscope (SEM) photograph of three beautifully formed crystals of the mineral analcime, NaAISi2O6*H2O, from Ischia, Italy. Each crystal displays a single form, a trapezohedron that reflects a high symmetry content in the isometric system. The trapezohedron is composed of 24 trapezium-shaped faces (from Gottardi, G., and Galli, E., 1985, Natural Zeolites, Springer-Verlag, New York; with permission)
The external form of crystals, or their morphology, is the outward expression of their internal ordered atomic arrangement. Figure 2.2 shows a packing model of the structure of halite, NaCI, with Na+ as small and Сl- as larger spheres.
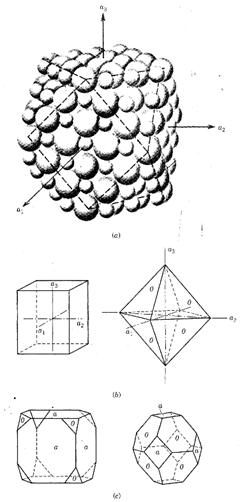
FIG. 2.2. (a) Packing model of halite with cubo-octahedral outline a1 a2, and a3 are reference axes that apply to the isometric system, (b) The geometric expression, in the external form of crystals, of the cube and the octahedron, (c) Two geometric combinations of a cube and an octahedron. The difference in these two crystals is the predominance of the cube in the left drawing and the predominance of the octahedron in the right drawing. The letters a and о are standard labels for cube and octahedral faces, respectively. See text for discussion
The outline of the model shows the presence of two crystal forms: a cube and an octahedron. The cube is the form with the six square faces perpendicular to the three axes (a1 a2 and a3); the octahedron is the form with eight triangular faces, each of which cuts off one of the eight corners of the cube. Upon careful inspection of the atomic packing shown in Fig. 2.2 it becomes obvious that the cube faces are underlain by planes that contain half Na+ and half Сl- ions.
On the other hand, the octahedral faces at the corners are underlain by planes containing only Сl-.
As such, different crystal planes represent differences in the underlying atomic environment. Figure 2.2 also shows the geometric appearance of two independent geometric forms, the cube and the octahedron; combinations of these forms are shown as well. In this early chapter in the text, without a basis in crystallography, suffice it to say that the study of crystal forms leads the investigator to the overall assessment of the symmetry content of the crystal at hand and subsequently to a classification of its symmetry as part of a crystal class (or point group).
The term form has a specific meaning in crystallography.
In its most familiar sense, form is used to indicate general outward appearance. In crystallography, however, external shape is denoted by the word habit, whereas form is used in a special and restricted sense. A form consists of a group of crystal faces, all of which have the same relation to the elements of symmetry (inherent in the crystal). Crystal habit, therefore, includes its general shape and irregularities of growth.
Although "perfect" crystals are shown in idealized crystal drawings in Chapter 6 and elsewhere in this text, most real crystals tend to be somewhat malformed. As such, their true symmetry is not immediately apparent; furthermore, the size of the equivalent faces may be unequal, and therefore the shape of the crystal as a whole appears distorted. Figure 2.3 shows some examples of crystal habit in the isometric system, and Fig. 2.4 illustrates how various divergent shapes and crystallographic forms may arise in the isometric system. The small cubic blocks that are used to build up the various shapes (in Fig. 2.4) are a schematic representation of the unit
cells of the underlying regular atomic arrangement. A unit cell is defined as the smallest unit of structure that if stacked indefinitely in three dimensions would form the whole structure.
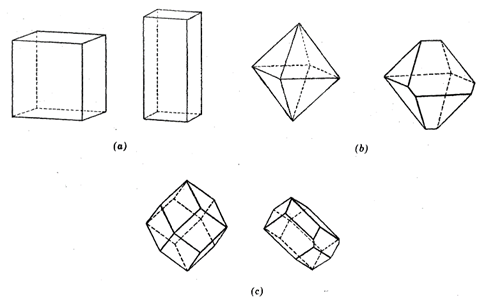
FIG. 2.3. Examples of perfect as well as distorted forms in the isometric system (a) Perfect cube and malformed cube, (b) Octahedron and asymmetric octahedron. (c) Dodecahedron and malformed dodecahedron
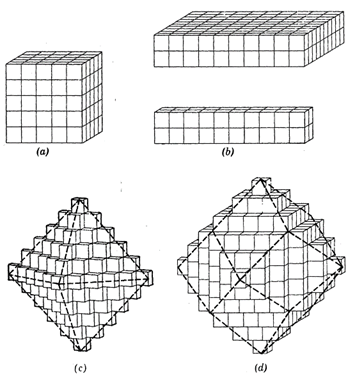
FIG. 2.4. Different external shapes are the result of the systematic stacking of cubic unit cells, (a) Perfect cube. (b) Distorted cubes. (c) Perfect octahedron, and (d) Perfect dodecahedron
Three commonly used terms express the quality of the development of the external faces on a crystal:
Euhedral. From the Greek roots eu, meaning good, and hedron, meaning plane; describing a mineral that is completely bounded by crystal faces and whose growth during crystallization was not restrained or interfered with by adjacent crystals or mineral grains.
Subhedral. From the Latin root sub, meaning less than; describing a crystal or mineral grain that is partly bounded by crystal faces and partly by surfaces formed against preexisting grains.
Anhedral. From the Greek root an, meaning without; for minerals that lack crystal faces and that may show rounded or irregular surfaces produced by the crowding of adjacent minerals during crystallization.
All the crystal drawings in this text are therefore of euhedral crystals, whereas the minerals that are intergrown, for example, in a granite, will tend to be subhedral and anhedral.
If mineral specimens display well-developed crystal forms, the form names are used to describe their outward appearance, as the following examples illustrate:
prismatic — for a crystal with one dimension markedly longer than the other two
rhombohedral — with the external form of a rhombo-hedron
cubic — with the external form of a cube
octahedral — with the external form of an octahedron
pinacoidal — with the pronounced development of one or more two-sided forms, the pinacoid.
Date added: 2022-12-31; views: 862;
