Cleavage, Parting, and Fracture
The properties of cleavage, parting, and fracture are the response of a crystalline material to an external force. When such a force is applied, a mineral is subjected to stress. If the internal structure of the crystalline substance is deformed, due to the stress, it is said to have undergone strain. As such, stress relates to the force applied, and strain to the resultant deformation. The strength of a crystalline material is a function of its bonding mechanism(s) and the presence (or absence) of structural defects.
The type of bonding (see Chapter 3) is of major importance in a mineral's reaction to an applied force. If a mineral contains structural defects along specific planes or directions, it will tend to deform along such features more easily than a mineral with a truly perfect (or better-ordered) structure. If the strain in the mineral is so great as to exceed its overall strength, it will break.
Many minerals have planar directions .in their structure that are systematically weaker than other directions. This is the result of planes in the crystal structure that are joined by fewer bonds per unit volume than are other planes in the structure, or are joined by weaker bonds.
Cleavage. Cleavage is the tendency of minerals to break parallel to atomic planes. Cleavage is a reflection of internal structure because within a structure the strength of chemical bonding is commonly different in different directions. This is especially well shown by layer structures (such as the various mica types and also graphite) in which the bonding within layers is very strong but between layers is much weaker. In these structures a perfect cleavage exists parallel to the layering.
Cleavage may be very well developed (perfect) in some crystals, as shown by the basal cleavage of micas, or it may be fairly obscure, as in beryl and apatite. In some minerals it is completely absent, as, for example, in quartz. Graphite, for example, has a well-developed platelike cleavage parallel to the . basal plane. Within these cleavage plates there is a strong covalent bond among the carbon atoms, but across the plates there are weak van der Waals bonds giving rise to the cleavage (see Fig. 3.26).
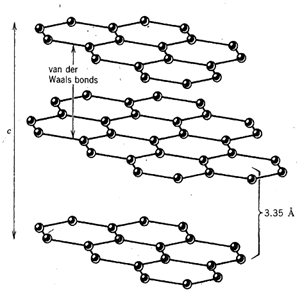
A weak bond is usually accompanied by a large interplanar spacing because the attractive force cannot hold the planes closely together. Diamond has only one bond type, the covalent bond, and its excellent cleavage parallel to the faces of the octahedron takes place along atomic planes having the largest interplanar spacing. The relationship between internal atomic structure and resultant cleavage directions is well shown by the structure image of a pyroxene in Fig. 2.15.
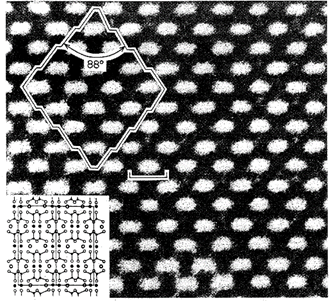
Fig.2.15. High-resolution transmission electron microscope (HRTEM) image of orthopyroxene showing possible cleavage surfaces a 88° to each other. The length of the bar is 8.8 A, which is the length of the b axis in orthopyroxene. The white regions in the image correspond to areas between M2 sites in the pyroxene structure. These light regions have relatively fewer atoms than the rest of the structure and hence have a low electron density. The insert shows the pyroxene structure at the same scale as the structure image. ’ (From Buseck, P. R. and lijima, S., 1974, High resolution electron microscopy of silicates
The planes of lowest bond density (i.e., the planes of lowest relative electron density) coincide with the two directions of the prism, the intersection of which is parallel to the c axis. The internal angle between these two cleavage directions is approximately 88°.
In describing a cleavage, its quality and crystallographic direction should be given. The quality is expressed as perfect, good, fair, and so forth. The direction is expressed by the name or Miller index (see p. 198) of the form that the cleavage parallels, such as cubic {001} (Fig. 2.16), octahedral (111), rhombohedral {1011}, prismatic {110}, or pinacoidal {001}. Cleavage is always consistent with the symmetry; thus, if one octahedral cleavage direction is developed, it implies that there must be three other symmetry-related directions. If one dodecahedral cleavage direction is present, it similarly implies five other symmetry-related directions. Not all minerals show cleavage, and only a comparatively few show it in an eminent degree, but in these it serves as an outstanding diagnostic criterion.

Parting. When minerals break along planes of structural weakness, they have parting. The weakness may result from pressure or twinning or exsolution; and, because it is parallel to rational crystallographic planes, it resembles cleavage. However, parting, unlike cleavage, is not shown by all specimens but only by those that are twinned or have been subjected to the proper pressure. Even in these specimens there are a limited number of planes in a given direction along which the mineral will break.
For example, twinned crystals part along composition planes, but between these planes they fracture irregularly. Familiar examples of parting are found in the octahedral parting of magnetite, the basal parting of pyroxene, and the rhombohedral parting of corundum (see Figs. 2.17 a and b).
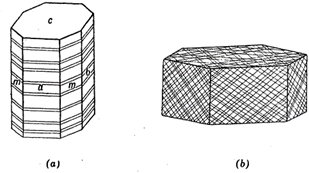
Fig. 2.17. (a) Basal parting, pyroxene, (b) Rhombohedral parting, corundum
Fracture. In some crystal structures the strength of the bonds is approximately the same in all directions. Breaking of such crystals generally will not follow a particular crystallographic direction. The way minerals break when they do not yield along cleavage or parting surfaces is their fracture. Fracture patterns can be distinctive and highly diagnostic in mineral identification.
Different kinds of fracture are designated as follows:
1. Conchoidal. The smooth, curved fracture resembling the interior surface of a shell (see Fig. 2.18). This is most commonly observed in such substances as glass and quartz.
2. Fibrous and splintery
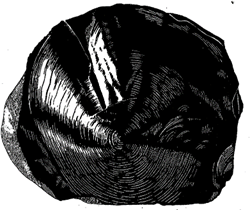
Fig. 2.18. Conchoidal fracture, obsidian
3. Hackly. Jagged fractures have sharp edges.
4. Uneven or irregular. Fractures produce rough and irregular surfaces.
Date added: 2022-12-31; views: 790;
