Tenacity. Specific Gravity
The_ resistance that a mineral offers to breaking, crushing, bending, or tearing—in short, its cohesiveness - is known as tenacity. The following terms are used to describe tenacity in minerals:
1. Brittle, A mineral that breaks and powders easily. This is characteristic of crystals with dominant ionic bonding.
2. Malleable. A mineral that can be hammered out into thin sheets.
3. Sectile, A mineral that can be cut into thin shavings with a knife.
4. Ductile. A mineral that can be drawn into a wire.
The characteristics described in 2, 3, and 4 (malleability, sectility, ductility) are diagnostic of materials held together by metallic bonding. The metallic bond conveys to crystalline substances the unique property of yielding to applied stress by plastic deformation. As shown in Fig. 3.24, metals are regarded as cations surrounded by a dense cloud of mobile electrons. These cations can, under stress, move past each other without setting up repulsive electrostatic forces. This atomic property is responsible for the physical behavior of metals under stress.
5. Flexible. A mineral that bends but does not resume its original shape when the pressure is released. Cleavage sheets of chlorite and talc are flexible, but they do not snap back to their original position after having been bent. In other words, their deformation is permanent. The bonding between the OH-rich layers in these silicates is by a combination of van der Waals and hydrogen bonds, whereas the bonding within the tetrahedral (Si-AI-O) sheets is a mixture of covalent and ionic bonding. The flexibility of the sheets is the result of slippage along OH-layers in the structure (see Fig. 11.35).
6. Elastic. A mineral that, after being bent, will resume its original position upon the release of the pressure. Sheets of mica can be bent, and they will snap back into their original position after the bending has stopped. In contrast to the structures of talc and chlorite, the mica structure contains K+-rich layers that exert a much stronger force on the sheets of Si—AI tetrahedra than do the hydrogen or van der Waals bonding mechanisms. The ionic bonding between K+. ions and the Si-AI tetrahedral sheets is responsible for the elasticity of mica (see Figs. 11,33 and 11.34).
Specific Gravity. Specific gravity (G) or relative density is a number that expresses the ratio between the weight of a substance and the weight of an equal volume of water at 4°C (this temperature is coincident with the maximum density of water). Thus, a mineral with a specific gravity of 2 weighs twice as« much as the same volume of water. The specific gravity of a mineral is frequently an important aid in its identification, particularly in working with fine crystals or gemstones, when other tests would injure the specimens. A listing of minerals arranged according to increasing specific gravity is given in Table 14,2 of Chapter 14.
The specific gravity of a crystalline substance depends on (1) the kind of atoms of which it is composed, and (2) the manner in which the atoms are packed together. In isostructural compounds (substances with identical structures) in which the packing is constant, those with elements of higher atomic weight will usually have higher specific gravities. This is well illustrated by the orthorhombic carbonates listed in Table 2.2, in which the chief differences are the atomic weight of the various cations.

In a solid solution series (see page 90), there is a continuous change in specific gravity (or density) with change in chemical composition. For example, the mineral olivine, (Mg, Fe)2Si04, is a solid solution series between forsterite, Mg2Si04 (G 3.3), and fayalite, Fe2Si04 (G 4.4). Thus, from determination of specific gravity one can obtain a close approximation of the chemical composition of an Mg-Fe olivine (see Fig. 12,4b). A similar relationship in an amphibole series is shown in Fig. 2.20.
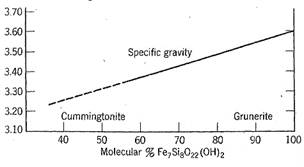
Fig. 2.20. Variation of specific gravity with composition in the monoclinic cummingtonite-grunerite series ranging if) composition from Fe2Mg5Si8O22(0H)2 to Fe7Si8022(0H)2. (After Klein, C., American Mineralogist, 1964
The influence of the packing of atoms on specific gravity is well illustrated in polymorphous compounds (substances of identical chemical composition but with different structures; see Table 4.2). In these compounds the composition remains constant, but the packing of the atoms varies. The most dramatic example is given by diamond and graphite, both elemental carbon. Diamond—with specific gravity 3.5—has a closely packed structure, giving a high density of atoms per unit volume; whereas in graphite—with specific gravity 2.23—the layers of carbon atoms are loosely packed.
Date added: 2022-12-31; views: 735;
