Types of Batteries. Primary and Secondary Batteries
There are two classes of batteries: primary and secondary. Primary batteries stop delivering electric current when their chemicals are expended. Secondary batteries—also called rechargeable batteries—can be recharged after use to restore them to their original charged condition. Batteries can also be categorized according to the electrolyte composition—that is, acid or base—or by their active chemical, such as lead, manganese, nickel, or zinc.
A battery's voltage is determined by the chemical energy stored in the electrode materials. Nominal voltage is the voltage level a battery is designed to have. A primary cell of the type used in a flashlight has a nominal voltage of 1 ½ volts. Most secondary batteries used to start automobiles are 12-volt batteries consisting of six 2-volt cells connected in a series.
Primary batteries use chemical reactions that are largely irreversible. They cannot be recharged efficiently. But a device called a battery charger may extend the life of certain types of primary batteries for a short time. It recharges a cell by passing a current through it in a direction opposite to that of the flow of electric current during discharge. Primary batteries require little maintenance and retain their charge even if stored for long periods. There are four main types of primary batteries: (1) alkaline, (2) lithium, (3) carbon-zinc, and (4) air.
Alkaline batteries are the most popular type of battery. They are excellent for high-power applications and extreme environmental conditions. The term alkaline refers to the electrolyte, which is an alkali (base).
The alkaline-manganese dioxide cell is among the most common alkaline batteries. The anode consists of zinc powder alloyed (mixed) with small amounts of other metals. The cathode is manganese dioxide, and the electrolyte is an aqueous (water-based) potassium hydroxide solution. Another type of alkaline battery is the silver oxide cell. Such a cell uses zinc powder as the anode, silver oxide as the cathode, and potassium hydroxide as the electrolyte. Silver cells produce more energy than alkaline-manganese dioxide cells, but they also cost more. Their use is limited to small button batteries that power calculators, watches, and cameras.
Lithium batteries are primary batteries with lithium metal anodes. A lithium cell produces more than twice the voltage of an alkaline cell of equal size. Lithium batteries are used in cameras, pacemakers, and watches.
Many different cathode materials and electrolytes are used in lithium batteries. One of the highest-energy cells is the lithium-thionyl chloride cell. In this cell, a liquid mixture of thionyl chloride and lithium tetrachloroaluminate acts as the cathode and electrolyte. Lithium metal serves as the anode. A porous carbon material serves as a cathode current collector, which receives electrons from the external circuit.
Carbon-zinc batteries, also called Leclanche (pronounced leh KLAHN SHAY) batteries, are packaged in a zinc can that serves as both a container and the anode.
The cathode is a mixture of manganese dioxide and carbon powder. The electrolyte is a mixture of zinc chloride and ammonium chloride dissolved in water. Carbonzinc batteries are the least expensive primary batteries. They can be used in flashlights, toys, or transistor radios.
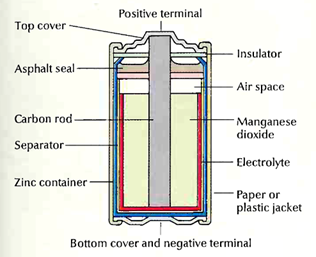
A carbon-zinc cell consists of a zinc container filled with sub-stances that react chemically to produce an electric current
Air batteries have a zinc anode and an aqueous potassium hydroxide electrolyte. The cathode includes a catalyst (substance that speeds a chemical reaction) that promotes a reaction of oxygen from the air with water to form negatively charged hydroxide ions. Air batteries are open to the atmosphere, and so practical applications are restricted to locations where the atmosphere is controlled. Air batteries are used in most hearing aids. This application is effective because a hearing aid rests in the ear canal, which generally maintains a constant humidity and temperature.
Secondary batteries have highly reversible electrode reactions. This feature allows such a battery to be recharged efficiently after use. Recharging reverses the chemical reactions at the anode and cathode, returning the battery to its original charged condition. This action can be performed hundreds of times. There are four common types of secondary batteries: (1 ) lead-acid, (2) nickel-cadmium, (3) metal hydride, and (4) lithium-ion.
Lead-acid batteries are used widely in automobiles. An automobile battery includes six 2-volt cells inside a plastic container. Each cell has two electrode structures called grids. A calcium-tin-lead alloy mesh filled with a spongy form of lead forms the negative grid. The positive grid consists of a lead-antimony alloy filled with lead dioxide. The electrolyte is sulfuric acid. In the energy-producing reaction, the spongy lead of the anode reacts with negatively charged sulfate ions in the electrolyte to produce lead sulfate. At the cathode, lead dioxide reacts with the electrolyte to produce lead sulfate.
Manufacturers have developed a lead-acid storage battery for automobiles that does not require the periodic addition of water. This maintenance-free battery is sealed except for a safety valve for venting gases. It uses grids made of lead-calcium-tin alloys and lasts much longer than a standard lead-acid battery. This alloy, unlike a lead-antimony alloy, minimizes water loss.
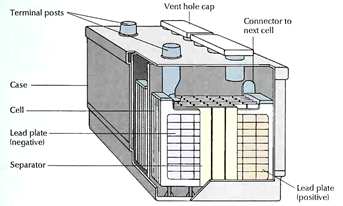
Parts of a lead-acid storage battery. Most lead-acid storage batteries have six cells. Each cell contains two sets of lead electrodes called plates. The plates are separated by plastic or rubber sheets. A solution of sulfuric acid, called the electrolyte, surrounds the plates. Each terminal post on the outside of the battery is connected to one set of plates. Vent holes in the case allow water to be added to the electrolyte and also permit gases produced in the cell to escape
Nickel-cadmium batteries, also called Ni-Cad batteries, are used in devices that require high power or a wide range of operating temperatures, such as cordless power tools. The cathode consists of nickel hydroxide and small amounts of cobalt. The anode is composed of cadmium metal. Porous nickel metal structures serve as the anode and cathode current collectors. The electrolyte is an aqueous potassium hydroxide solution. A Ni-Cad battery has a nominal voltage of 1.3 volts.
Metal hydride batteries use a special metal alloy that absorbs hydrogen as the anode's active material. Such an alloy can absorb over a thousand times its own volume of hydrogen. The cathode is nickel hydroxide. The anode is an alloy of nickel and lanthium, or of transition metals, which include chromium, iron, nickel, titanium, vanadium, and zirconium. The electrolyte is aqueous potassium hydroxide. Its composition does not change during the reaction. Metal hydride batteries are used in camcorders, cellular telephones, and computers. A metal hydroxide cell can produce up to 1.35 volts.
Lithium-ion batteries produce and store electrical energy by reversibly shuttling lithium ions between the anode and the cathode. The active materials have crystal structures that permit lithium ions to enter and exit without altering the structure. In most lithium-ion batteries, the anode is a carbon-based material, such as graphite or coke, and the cathode is a cobalt, manganese, or nickel oxide that includes lithium. The nominal voltage is 4.1 volts. Lithium-ion batteries are used in camcorders, cellular telephones, laptop computers, and reserve power supplies.
How Batteries Work
Battery is a device that converts chemical energy directly into electrical energy. Batteries are used to power a variety of devices, including radios, automobile starters, and electronic equipment on satellites. Tiny batteries, such as those used in hearing aids, are less than 0.2 inch (6 millimeters) in diameter and weigh 1/100 ounce (0.3 gram).
The largest batteries, such as those that power submarines, can weigh over 100 tons (90 metric tons). A backup system called an uninterruptible power supply (UPS) uses batteries to maintain power during a utility power failure. A UPS enables computers, emergency lights, and hospital equipment to continue operating if a power loss occurs.

Batteries have many uses. The small "button " in the foreground powers a wrist watch. The large battery at the rear provides the power to start an automobile. The other batteries shown operate radios, tape recorders, toys, and other items
Batteries are manufactured in a wide range of sizes and shapes. The International Electrotechnical Commission, based in Geneva, Switzerland, sets standard sizes and minimum performance specifications for many batteries. The basic dimensions of such common sizes as D, C, AA, and AAA are thus consistent from manufacturer to manufacturer, regardless of the country in which the batteries are made. Such standardization helps ease world trade in electrical and electronic products.
How batteries work. The fundamental unit of a battery is the electrochemical cell Each such cell has all the chemicals and parts needed to produce an electric current. The word battery actually refers to a group of connected cells. But the term is often applied to a single cell.
An electrochemical cell has two structures called electrodes. Each electrode is composed of a different chemically active material. The electrodes can be connected to external terminals, which, when joined by wire, complete a closed external circuit. When a cell is connected to such a circuit, an oxidation reaction at one electrode, called the anode or negative electrode, releases electrons to the circuit. At the other electrode, called the cathode or positive electrode, a reduction reaction occurs, and the electrode receives electrons from the external circuit. See Oxidation; Reduction.
Inside the cell, the anode and the cathode are separated by an electrolyte. An electrolyte is an ionic conductor, through which ions (electrically charged atoms) may move freely. The electrolyte is usually an acid, a base (substance that neutralizes acid), or a salt. When a closed external circuit is connected to the cell, positive and negative ions in the electrolyte move inside the cell to complete the circuit. The positive ions move from the anode to the cathode, and the negative ions move from the cathode to the anode. In this way, an electric current flows through the circuit. An electrical device, such as a light bulb, motor, or radio, can be joined to the circuit to enable the current to do useful work.
Most cells include a separator, a porous, nonconducting barrier that absorbs the electrolyte and prevents direct contact between the two electrodes. If the anode and cathode were to touch, the energy-producing reactions would occur inside the cell, and a current would not be available to do useful work outside the cell.
Date added: 2022-12-31; views: 2116;
