Magnetism. Radioactivity. Piezoelectricity
Many minerals experience no attraction for a magnetic field (these are referred to as diamagnetic), whereas other minerals may be drawn to a magnetic field (these are known as paramagnetic). The origin of magnetism in minerals is discussed on pp. 164 to 167. Because minerals show a wide range of magnetic susceptibility they can be separated from each other by strong electromagnets.
Magnetic separation by a Franz Isodynamic Separator is a common procedure in industrial, and research laboratories. Such a technique separates minerals that are paramagmetic from those that are diamagnetic. On a commercial scale, electromagnetic separation is used to separate ore minerals from gangue (waste).
In a mineralogy laboratory the student will encounter two common highly magnetic (paramagnetic) minerals, magnetite and pyrrhotite. That is, both are easily attracted to a small hand magnet. Both are opaque and may occur as minor constituents in a wide range of mineral associations and rock types. Even if present in small quantities, or with small-sized grains, removing one or several grains from a specimen with a needle or a pocketknife will allow you to test the magnetism of individual grains. Magnetite is very strongly attracted to a magnet, pyrrhotite less so.
Radioactivity. Minerals containing uranium and thorium will continually undergo decay reactions in which radioactive isotopes of U and Th form various daughter elements and also release energy in the form of alpha and beta particles and gamma radiation. The radiation produced can be measured in the laboratory or in the field using a Geiger counter or a scintillation counter. A radiation counter, therefore, is helpful in the identification of U- and Th-containing minerals. Examples are uraninite, pitchblende, thorianite, and autunite. The origin of radioactivity is discussed on pp. 167 to 168.
Solubility in HCl. The positive identification of carbonate minerals is much aided by the fact that the carbon-oxygen bond of the (C03) group in carbonates becomes unstable and breaks down in the presence of hydrogen ions available in acids. This is expressed by the reaction
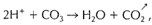
which is the basis for the "fizz" test with dilute hydrochloric acid. Calcite, aragonite, witherite, and strontianite as well as Cu-carbonates show bubbling or effervescence (fizz) when a drop of dilute HCI is placed on the mineral. The fizz is the result of the release of C02. Other carbonates such as dolomite, rhodochrosite, magnesite, and siderite will show effervescence only in hot HCI.
Piezoelectricity. The conduction of electricity in crystals is related to the type of chemical bonding. Minerals with pure metallic bonding, such as the native metals, are excellent electrical conductors, whereas those in which the bonding is partially metallic, as in some sulfide minerals, are semiconductors. lonically or covalently bonded minerals are usually nonconductors. In some nonconducting minerals it is possible to induce electrical charges using directed pressure (piezoelectricity) or temperature (pyroelectricity).
Piezoelectricity is extensively used in industrial applications for the control of radio frequencies in electronic circuits. Piezoelectricity occurs only in those crystalline substances that lack a center of symmetry (see Table 5.3). Of the 32 crystal classes (point groups; see Chapter 6), 21 have no center of symmetry, and of these all but one, the gyroidal class (with point group symmetry 432), has at least one polar crystallographic axis with different crystal forms at opposite ends of that specific axis. (A polar axis is a crystallographic concept.
A crystal is said to have a polar axis if one direction of an axis is not related by symmetry to the opposite direction along that same axis. Such a crystal is referred to as noncentrosymmetric, meaning that it lacks a center of symmetry). If pressure is exerted at the ends of a polar axis, a flow of electrons toward one end produces a negative electrical charge, whereas a positive charge is induced at the opposite end. This is piezoelectricity, and any mineral crystallizing in one of the 21 classes with polar axes should show it. However, in some minerals the charge developed is too weak to be detected.
The property of piezoelectricity was first detected in quartz in 1881 by Pierre and Jacques Curie, but nearly 40 years passed before it was used in a practical way. Toward the end of World War I it was found that sound waves produced by a submarine could be detected by the piezoelectric current generated when they impinged on a submerged quartz plate. The device was developed too late to have great value during the war, but it pointed the way to other applications. In 1921 the piezoelectric property of quartz was first used to control radio frequencies, and since then millions of quartz plates have been used for this purpose.
When subjected to an alternating current, a properly cut slice of quartz is mechanically deformed and vibrates by being flexed first one way and then the other; the thinner the slice, the greater the frequency of vibration. By placing a quartz plate in the electric field generated by a radio circuit, the frequency of transmission or reception is controlled when the frequency of the quartz coincides with the oscillations of the circuit. The tiny quartz plate used in digital and analog quartz watches serves the same function as quartz oscillators used to control radio frequencies.
That is, it mechanically vibrates at a constant frequency that is a function of the plate's thickness and the crystallographic orientation of the slicing within the original quartz crystal. This quartz frequency controls accurately the radio frequency of the electronic circuit in the watch. This circuit counts the crystal frequency and provides the digital time display of the watch. Figure 2.22 is an illustration of the basic schematic of a liquid crystal watch for the display of hours and minutes.
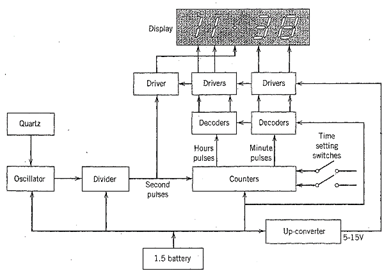
Fig. 2. 22. Block diagram of a liquid crystal watch. (Redrawn after Burfoot, J. C. and Taylor, G. W., 1979, Polar Dielectrics and Their Applications. University of California Press, Berkeley, Calif. Copyright © 1979, Jack Burfoot and George Taylor.)
The quartz crystal controls an oscillator circuit, which, in turn, generates pulses of one second in time. These "second" pulses are counted to produce "minute" and "hour" pulses. Each of these pulses is decoded to provide proper outputs for the digital watch display. Powered by a 1.5 V silver oxide battery, a quartz plate vibrates approximately 100,000 times per second. An inexpensive quartz watch today is more accurate than the best-made mechanical watch, and precision-manufactured quartz clocks are accurate to within one second per ten years.
The piezoelectric property of tourmaline has been known almost as long as that of quartz, but compared with quartz, tourmaline is a less effective radio oscillator and is rare in occurrence. Nevertheless, small amounts of it are used today in piezoelectric pressure gauges. In tourmaline, which is hexagonal in symmetry, the vertical c axis is a polar axis. Plates cut normal to this direction will generate an electrical current when subjected to a transient pressure.
The current generated is proportional to the area of the plate and to the pressure. Tourmaline gauges were developed to record the blast pressure of the first atomic bomb in 1945 and since then have been used by the United States with each atomic explosion. Lesser pressures also can be recorded by them, however, such as those generated by firing a rifle or by surf beating on a sea wall.
REFERENCES AND SELECTED READING:
- Hochieitner, R. 1994. Minerals, classifying and collecting them. Barron's Educational Series Inc. New York: Hauppauge.
- Hurl but, C. S., Jr., and W. E. Sharp. 1998. Dana's minerals and how to study them. 4th ed. New York: Wiley.
- Keffer, F. 1967. The magnetic properties of materials. Scientific American 217: 222-38.
- Klein, С. 1994. Minerals and rocks: Exercises in crystallography, mineralogy, and hand specimen petrology. Rev. ed. New York: Wiley.
- Loeffler, В. M., and R. G. Burns. 1976. Shedding light on the color of gems and minerals. American Scientist 64: 636-47.
- Simon and Schuster's guide to rocks and minerals. 1978. New York: Simon and Schuster.
- Zussman, j. 1977. Physical methods in determinative mineralogy. New York: Academic Press
Date added: 2022-12-31; views: 1010;
