Hardness. Mohs Hardness Scale and Additional Observations
The resistance that a smooth surface of a mineral offers to scratching is its hardness (designated by H). The degree of hardness is determined by observing the comparative ease or difficulty with which one mineral is scratched by another, or by a file or knife. The hardness of a mineral might then be said to be its "scratchability."
The evaluation of hardness is the assessment of the reaction of a crystal structure to stress without rupture (cleavage, parting, and fracture are various forms of rupture). In metallic-bonded crystals that can flow plastically, scratching results in a groove. However, brittle materials with ionic or covalent bonds react to a hardness test by microfracturing (rupture on a very fine scale). The effect of ionic size and charge in ionically bonded structures is discussed in Chapter 3 (see Fig. 3.19).
This illustrates how different chemical compounds with the same internal structure increase in hardness with decreasing ionic size and increasing ionic charge. In relating the hardness of a crystal structure to its bonding, it must be noted that the structure's overall strength is a composite of all of its bond types, whereas the hardness of that same structure is an expression of its weakest bonding. For example, in silicates, all of which are based on various arrangements of Si04 tetrahedra, the hardness ranges from 1, as in talc, to - 7, as in quartz, and to 8, as in topaz. Such a variation suggests that hardness is not a function of Si-O bonding, but rather of the other bond types present in the structure.
In talc the basal silicate layers are held together by weak van der Waals and/or hydrogen bonds; in quartz there is a uniform bond strength within a relatively dense network of Si04 tetrahedra; and in topaz there are somewhat weaker AI-(F, OH) bonds.
A series of 10 common minerals was chosen by the Austrian mineralogist F. Mohs in 1824 as a scale, by comparison with which the relative hardness of any mineral can be told. The following minerals, arranged in order of increasing hardness, comprise what is known as the Mohs scale of hardness:

These minerals are arranged in an order of increasing relative hardness. Their hardness can also be measured by more quantitative techniques than a scratch test, and this leads to an absolute hardness scale as shown in Fig. 2.19. The relative position of the minerals in the Mohs scale is preserved, but corundum, for example, is two times as hard as topaz and four times harder than quartz.
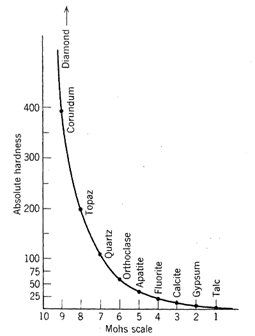
Fig. 2.19. Comparison of Mohs relative hardness scale and absolute measurements of hardness
Talc, number 1 in the Mohs scale, has a structure made up of plates so weakly bound to one another that the pressure of the fingers is sufficient to slide one plate over the other. At the other end of the scale is diamond, with its constituent carbon atoms so firmly bound to each other that no other mineral can force them apart to cause a scratch.
In order to determine the relative hardness of any mineral in terms of this scale, it is necessary to find which of these minerals it can and which it cannot scratch. In making the determination, the following should be observed:
1. Sometimes when one mineral is softer than another, portions of the first will leave a mark on the second that may be mistaken for a scratch. Such a mark can be rubbed off, whereas a true scratch will be permanent.
2. The surfaces of some minerals are frequently altered to material that is much softer than the original mineral. A fresh surface of the specimen to be tested must therefore be used.
3. The physical nature of a mineral may prevent a correct determination of its hardness. For instance, if a mineral is pulverulent, granular, or splintery, it may be broken down and apparently scratched by a mineral much softer than itself. It is always advisable when making the hardness test to confirm it by reversing the order of procedure; that is, do not only try to scratch mineral A by mineral B, but also try to scratch В by A.
Several materials serve in addition to the above scale (see Table 2.1): the hardness of the fingernail is a little over 2, a copper coin about 3, the steel of a pocketknife a little over 5, window glass 5 ½ and the steel of a file 6 1/2 . With a little practice, the hardness of minerals under 5 can be quickly estimated by the ease with which they can be scratched with a pocketknife.
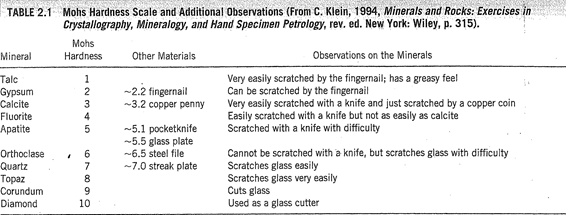
TABLE 2.1. Mohs Hardness Scale and Additional Observations (From C. Klein, 1994, Minerals and Rocks: Exercises in Crystallography, Mineralogy, and Hand Specimen Petrology, rev. ed. New York: Wiley, p. 315)
Hardness is a vectorial property. Thus, crystals may show varying degrees of hardness, depending on the directions in which they are scratched. The directional' hardness differences in most common minerals are so, slight that, if they can be detected at all, it is only through the use of delicate instruments. Two exceptions are kyanite and calcite. In kyanite, H = 5 parallel to the length, but H = 7 across the length of the crystal. The hardness of calcite is 3 on all surfaces except the basal pinacoid {0001}; on this form it can be scratched by the fingernail and has a hardness of 2.
Because there is a general link between hardness and chemical composition, the following generalizations can be made.
1. Most hydrous minerals are relatively soft (H < 5).
2. Halides, carbonates, sulfates, and phosphates are also relatively soft (H < 5 1/2).
3. Most sulfides are relatively soft (H < 5) with pyrite being an exception (H < 6 to 6 1/2).
4. Most anhydrous oxides and silicates are hard (H>5 1/2)
Because hardness is a highly diagnostic property in mineral identification, most determinative tables use relative hardness as a primary sorting parameter, as is done in the Determinative Table 14.1.
Date added: 2022-12-31; views: 1010;
