Mercury in the Aquatic Environment. Mercury Health Effects
Airborne Hg(0) is eventually oxidized to mercuric mercury [Hg(II)], which can sorb onto aerosols or particulates in air and is highly soluble in water. It is thus deposited in the ocean and on land, ultimately making its way into lakes, rivers, streams, and wetlands (Figure 1).
The total dissolved Hg concentration in open ocean ranges from 0.1 to 0.5ngL-1 and from 1 to 10ngL-1 in lakes or terrestrial aquatic systems. Recent studies estimate that the total amount of anthropogenic Hg present in the global oceans is about 58 000 ± 136 000 metric tons, with almost two-thirds residing in water shallower than a 1000 m.
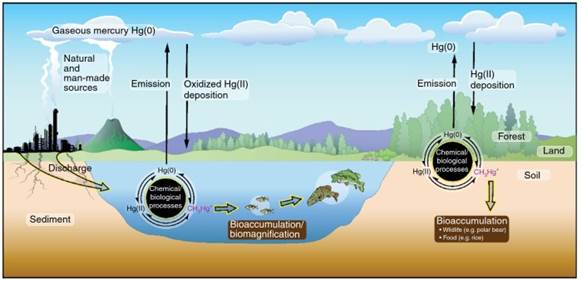
Figure 1. Illustration of global mercury (Hg) cycling, transport, and transformation leading to the bioaccumulation and biomagnification of Hg in biota. Note that Hg(0), Hg(II), and CH3Hg+ denote metallic elemental Hg, inorganic mercuric Hg2+ species, and methylmercury, respectively
The upper 100 m of the oceans contain twice the Hg than they did a century ago. This finding suggests that anthropogenic perturbations to the global Hg cycle have led to an approximately 150% increase in the amount of Hg in thermocline waters and have tripled the Hg content of surface waters compared with preanthropogenic conditions.
As a result, mercury concentrations in marine biota are likely to increase slowly for decades to centuries, even without an increase in atmospheric emissions. Similarly, mercury levels in freshwater ecosystems will likely continue to increase from atmospheric deposition and remobilization from lands and soils, even in regions where atmospheric concentrations have decreased because of emission controls.
As detailed in Section 3.1, natural processes in aquatic systems convert less toxic elemental Hg(0) and inorganic Hg(II) into much more toxic organometallic MeHg, a neurotoxin, which concentrates and bioaccumulates in various sources of food, such as fish and rice, potentially endangering humans and other wildlife.
Mercury bioaccumulation is a process by which organisms (e.g. fish, aquatic invertebrates, and mammals) acquire Hg more rapidly than their bodies can expel it, causing the amount ofHgto accumulate overtime. Biomagnification is the incremental increase in Hg concentrations with increasing trophic levels (or at each level of a food chain).
This phenomenon occurs because the food source for organisms higher in the food chain is progressively more concentrated in Hg, thus Hg concentrations are magnified at the top of the food chain (Figure 1). In general, the biomagnification effect increases with the age and trophic level of the organism. As a result, Hg concentrations in predatory fish such as swordfish and tuna can be millions of times higher relative to surrounding waters.
More than 90% of the Hg found in fish and shellfish is present in the form of MeHg. Adding to this problem is the fact that Hg accumulates in the muscle tissue of fish. Unlike organic contaminants, such as polychlorinated biphenyls, which concentrate in fish skin and fat, mercury strongly binds to proteins resulting in high muscle tissue concentrations and cannot be removed during food preparation.
Mercury Health Effects. Human exposure to Hg has been implicated with three major pathways: (i) MeHg exposure as a result of food consumption such as fish or rice (particularly in countries where rice is a staple food), (ii) exposure to inorganic Hg(II) from contaminated water, and (iii) inhalation of gaseous Hg(0) emitted from various sources, such as metallic Hg, dental amalgams, and ambient air. The potential toxicity effects from gaseous Hg(0) and Hg(II) are generally less severe, unless the body is subjected to prolonged exposure at high concentrations.
As a vapor, Hg(0) is rapidly absorbed into the blood stream when inhaled. The most common form of direct exposure for humans, however, is through consumption of fish or other seafood contaminated with MeHg. Once ingested, about 95% of the MeHg is absorbed through the gastrointestinal tract. In some developing countries, rice consumption, rather than fish, is the primary exposure pathway.
Rice grains can accumulate a significant amount of MeHg from paddy soils, which harbor microbes that can convert inorganic Hg to MeHg. Therefore, given the broad range of MeHg exposure scenarios the majority of the world’s population has at least a small amount of Hg in their bodies.
Mercury, particularly MeHg, can potentially damage the central nervous system and cause neuropathies and developmental disorders, especially in children. Symptoms from the damage are also known as Minamata disease. In more severe cases, mercury can cause irreversible brain damage; loss of peripheral vision; damage to the gastrointestinal tract; and impaired speech, hearing, walking, and coordination of movement.
Two well-documented cases of MeHg poisoning include Minamata Bay, Japan, which was the result of an industrial point-source release of MeHg in 1956 and Iraq in 1971 from consumption of MeHg-treated wheat grains. In each case, hundreds of people died, and thousands were affected, many with permanent damage. The greatest effects appeared in children of women who had consumed contaminated bread during pregnancy.
Chronic exposure to Hg has the potential to cause kidney damage at levels above the maximum contaminant level (MCL) of 2 pg L-1 for inorganic Hg(0) and Hg(II) by the U.S. Environmental Protection Agency (EPA). Mercury contamination can also seriously impact ecosystems, including reproduction of birds and predatory mammals. MeHg concentration in natural water is extremely low, usually <1 ngL-1, even in Hg-contaminated systems; see Sections 3 and 4 for additional details. The MCL for MeHg is based on consumption of fish tissue, the primary human exposure pathway, and is set at 0.3 mg kg-1 by the EPA.
Date added: 2023-10-03; views: 705;
