Mercury Biogeochemical Transformations. Chemical Reduction and Oxidation in the Dark
Mercury has three oxidation states: Hg(0) (elemental mercury), Hg+ (mercurous mercury), and Hg2+ (mercuric mercury). Mercuric Hg2+ is the most common oxidation state found in natural soil and aquatic environments, whereas gaseous Hg(0) primarily exists in the atmosphere. However, free Hg2+ ions do not usually exist in natural water but form complexes with various organic and inorganic counter ions or ligands, such as hydroxide (OH-), chloride (Cl-), sulfide (S2-) anions, and dissolved organic matter (DOM), because of their strong binding affinities.
These complexes are generally referred to as Hg(II) chemical species. Mercurous Hg+ or Hg(I) compounds mostly exist in the form of dimeric cations (Hg22+), which can form numerous inorganic and organic compounds. However, dimeric Hg22+ is unstable under circumneutral pH conditions and can disproportionate to form Hg(0) and Hg2+ in solution.
Similar to many redox-sensitive metal ions, mercury readily undergoes chemical reduction or oxidation depending on the redox potential of the reductant or oxidant. Oxidation of Hg(0) to Hg(II) can occur in the atmosphere, natural water [26], and soil.
Atmospheric oxidation is a major pathway leading to Hg deposition, as Hg(II) is scavenged by precipitation and/or sorption onto aerosols or small particulates in the atmosphere (Figure 1). In natural water and sediments, Hg redox transformations can be mediated by both biological and chemical (including photochemical) processes, as detailed in Sections 2.1-2.3.
Understanding the reduction and oxidation or redox transformations of Hg is critically important because the properties and chemical behavior of Hg strongly depend on its oxidation state and chemical form. Each of these factors influences the reactivity, mobility, and availability of Hg for microbes to produce MeHg.
Chemical Reduction and Oxidation in the Dark. Natural DOM or humic substances exist ubiquitously in water and sediments and are perhaps among the most prevalent reducing agents with the ability to convert Hg(II) to Hg(0) in aquatic systems. The mechanism of Hg(II) reduction by DOM is attributed to the presence of semiquinone moieties or functional groups on DOM acting as reductants.
These functional groups are also known to reduce metal ions such as ferric iron [Fe(III)] and uranyl (UO22+) to form ferrous iron [Fe(II)] and uraninite (UO2) species. However, DOM is a relatively weak reductant and unable to reduce Hg(II) complexes that are associated with thiols (-SH), such as cysteine and glutathione.
In laboratory studies, stannous chloride (SnCl2) is among the most commonly used chemical reductant, and it has the ability to reduce simple organic and inorganic Hg(II) species, such as dissolved Hg2+, Hg(OH)2, HgCl2, and Hg-acetate or Hg-cysteine complexes to form elemental Hg(0). However, SnCl2 is incapable of reducing strongly bound Hg(II) species such as MeHg and some complexed with DOM or particulate organic matter in water. Stronger reducing agents such as sodium borohydride are necessary to reduce MeHg and strongly bound Hg(II)-DOM complexes.
Importantly, natural DOM not only reduces Hg(II) but also oxidizes Hg(0) in the dark in water. In other words, DOM plays a dual functional role in Hg reduction and oxidation because of the presence of multiple functional groups on DOM. Here, the oxidation of Hg(0) by DOM is controlled by reduced sulfur or thiol (-SH) functional groups, which are a minor component of DOM compared to the more abundant semiquinone moieties (Figure 2).
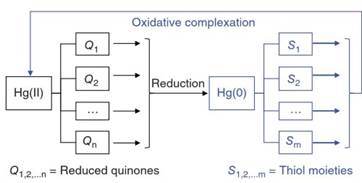
Figure 2. Dual functional roles of naturally dissolved organic matter (DOM) in which the reduced quinone or semiquinone (Q) functional groups reduce Hg(II) to elemental Hg(0), whereas the thiol (-SH) or thiolate functional groups on DOM promote oxidation of Hg(0)
This reaction is referred to as “thiol-induced oxidative complexation" because of exceptionally strong binding affinities between Hg and thiol functional groups. The reaction is thus favored under reducing environments, where DOM can also be reduced by anaerobic microbes, such as iron-reducing bacteria and sulfate-reducing bacteria (SRB), leading to increased thiols or thiolate functional groups on DOM.
Whether Hg reduction or oxidation dominates in an aquatic system will depend on the redox state of Hg [either Hg(II) or Hg(0)] and its ratio to DOM. At relatively low dissolved organic carbon (DOC):Hg ratios, Hg(II) reduction likely dominates because of the availability of abundant semiquinone moieties (or reducing equivalents) but limited thiols on DOM. At a relatively high DOC:Hg ratio, however, Hg-DOM complexation (e.g. with thiols) dominates and prevents Hg(II) reduction but promotes Hg(0) oxidation, if present.
These reactions are particularly important in anoxic water and sediments, where microbial reduction of DOM and methylation are active and can thus result in dynamic changes of Hg chemical species. Oxidation of Hg(0) by thiols can scavenge dissolved Hg(0) and transform it into thiol-bound Hg(II), which is shown to affect microbial methylation. Changes in redox conditions can also alter the redox state of sulfur and quinone or hydro- quinone moieties and therefore the binding, reduction, and oxidation of Hg.
Dissolved elemental Hg(0) is also readily oxidized by a wide variety of chemical oxidants, among which permanganate (MnO4-) and bromine chloride (BrCl) are the most commonly used in laboratory preparations. Both BrCl and MnO4- are strong oxidants and are often used to determine the total Hg in aqueous samples by oxidizing all the Hg species to Hg(II) in aqueous samples and subsequently reducing Hg(II) to Hg(0), which is analyzed via a vapor-phase Hg(0) analyzer.
Although thermodynamically favorable, Hg(0) oxidation by dissolved oxygen in water is slow. It is quite stable in pure water under oxic conditions, but the presence of halides such as Clin brackish water and seawater can greatly increase the rate of Hg(0) oxidation.
Date added: 2023-10-03; views: 750;
