Fibers, Synthetic and Semi-Synthetic
Silk has long been prized as a fiber for fashion, and Robert Hooke proposed artificial silk at least as early as 1665. The invention of the electric light bulb in 1879 created a new incentive for an improved fiber: a very uniform carbon fiber was required for lamp filaments. In 1883, Joseph W. Swan invented a process of squirting a nitrocellulose solution through a dye into a coagulating bath of water and alcohol. A denitration technique was used to make the fiber less flammable.
In 1885, he exhibited the material at the Inventions Exhibition as “artificial silk.’’ Although Swan was the first to spin fibers from a nitrocellulose solution, the first commercial production of artificial silk took place in France in 1884 by Louis de Chardonnet. De Chardonnet’s product, however, failed commercially as he did not denitrate the fibers to reduce their flammability, with disastrous results. In the first quarter of the twentieth century, the Chardonnet process gradually disappeared.
In Germany, Max Fremery and Johan Urban produced good quality fibers by using solutions of cellulose in a copper oxide and ammonia mixture. In 1899, production started in the Vereinigte Glanzstoff Fabriken AG. In England in 1884, Charles Cross and Edward Bevan discovered that alkali-cellulose could be converted into cellulose-xanthogenate, which could be dissolved in sodium hydroxide. This solution was called ‘‘viscose,’’ from which fibers could be pulled. Together with Clayton Beadle, they established the Viscose Syndicate Ltd. at Kew. Viscose fibers were of very good quality, and gradually the viscose process became the main process for producing artificial silk.
After World War I there was no use for vast amounts of cellulose acetate lacquers that had been used for doping fabric-covered aircraft. Henry and Camille Dreyfuss used these lacquers to produce fibers. In 1921, they introduced acetate fibers to the U.S. ‘‘Acetate’’ made great inroads on the market.
After World War I it was very important to show that cellulose fibers were not merely a ‘‘surrogate’’ for silk, and the name ‘‘rayon’’’ was introduced in 1924 and gradually accepted worldwide. Though rising hemlines created a demand for cheaper alternatives to silk stockings, rayon ‘‘bagged’’ at the ankles and in the 1930s and 1940s uses turned more to industrial products. One of the main improvements was a ‘‘hot stretch’’ process to increase the strength of rayon.
In this way rayon could replace cotton in tire cords. Although the basic features of the three processes to produce rayon fibers (viscose—by far the most significant process by volume—acetate, and cuprammonium) did not change much, efficiency of production continued to increase. Between 1930 and 1940, DuPont’s manufacturing costs of a pound of viscose fiber fell nearly 60 percent. Until the recession of 1929, the rayon industry was a golden business.
However, the basic science of rayon was not understood. Chemists explained the characteristics of these materials by postulating forces between circular molecular structures. Most important in challenging these established concepts was Hermann Staudinger. He claimed in 1920 that normal molecular bonds could explain the products of polymerization reactions.
Compounds, which precipitated during these reactions, could be explained by assuming that hundreds of molecules merged into “macromolecules.’’ The work of Wallace H. Carothers, at E.I. DuPont de Nemours, was crucial for the acceptance of Staudinger’s concepts. From 1928 onward, Carothers proved the existence of macromolecules by synthesizing new, long-chain molecules. Julian Hill, one of his research group members, discovered that he could draw fibers from the melt of these materials. In 1934, Carothers’s group succeeded in making a good- quality polyamide 6,6 fiber.
Technological problems were enormous: the process for spinning the fibers differed greatly from any conventional process. At the end of 1938, DuPont launched the fiber as Nylon. Nylon stockings were an overwhelming commercial success until it was rationed to replace silk in parachutes and to reinforce airplane tires. In Germany, Paul Schlack had succeeded in producing a Nylon-like fiber by the end of the 1930s. The fiber was often called Nylon 6 as opposed to DuPont’s Nylon 6,6. After the war, the nylon 6 patents of IG Farben were confiscated. Many corporations started Nylon 6 production, as the technology was freely available.
During the war, research on new fibers continued. The most important of the new fibers were acrylic fibers and polyester fibers, including terylene discovered in Britain by John R. Whinfield and James T. Dickson in 1941. ICI and DuPont started producing polyester fiber (terylene and dacron) in the early 1950s. Polyester fiber had very good mechanical properties and a better light stability than nylon. Because of its very good resilience, the main application of this fiber was initially in ‘‘wash and wear’’ textiles.
In 1942, IG Farben found a suitable solvent to spin acrylic fibers, and after World War II, they were commercialized by Bayer, DuPont and Monsanto. Acrylic fibers had comparable properties to wool, and the chemical, thermal and light stability of the fiber was rather good. Acrylic fibers were spun by a new spinning process: after spinning, the solvent evaporated when the fibers were led through a gas stream in a spin tower.
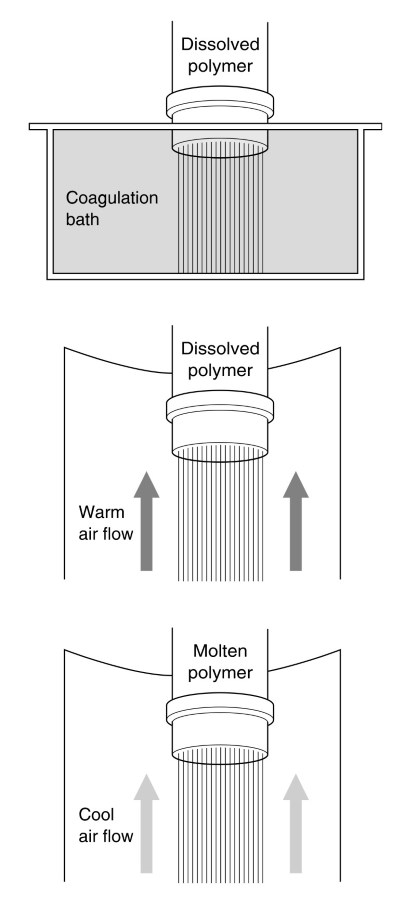
Figure 2. Wet spinning, dry spinning, melt spinning
This process is called dry spinning. Nylon and polyester were spun by a melt spinning process, which implies that the polymers are molten before spinning, and not dissolved. Rayon was produced by wet spinning, which implies that the polymer is dissolved. However, the solvent is later removed from the fiber in a bath.
Polypropylene fiber was developed at the end of the 1950s, based on a breakthrough in polymer science at the beginning of the 1950s in which ordered polyethylene and polypropylene polymers could be made catalytically. Polypropylene fiber was excellent for ship ropes, as its strength was high and it floated. Polyethylene was also used for fibers after DuPont invented the ‘‘flash spinning’’ technique by which very cheap, but low-quality polyethylene fibers could be spun. Polyethylene fibers were used to form a sheet-like wrapping material.
Particularly in the 1950s and 1960s, synthetic fibers conquered large shares of the market. Production efficiency and fiber properties increased considerably. For example polyester-spinning speeds increased from 500 meters per minute in 1961 to 4000 meters per minute in 1975, allowing fiber prices to drop dramatically in these years. Improved quality opened new markets. For example, nylon was good enough to be used in car tires after World War II, although tires reinforced with nylon were subject to so-called ‘‘flat spotting’’; they had a flat spot when the car had been parked for a while.
At the beginning of the 1950s, scientists at DuPont achieved a breakthrough in polymerization. Their new method did not involve heating of intermediates. A lot of new polymers were made which could not be made before because the intermediates could not withstand heating. By this method, DuPont scientists developed the spandex fiber Lycra®. A high temperature-resistant fiber, Nomex®, was made based on a wholly aromatic polyamide. Similar fibers were introduced in Japan as Conex® and in the USSR as Fenilon®.
Other companies also started researching DuPont’s low temperature polycondensation method. Celanese developed very heat-resistant and comfortable polybenzimidazole fibers. Protective clothing became more important when three of the Apollo I astronauts burned in their spacecraft in an accident during launching in January 1967.
The 1950s and 1960s were the glorious days of the fiber industry. Growth rates and profits were high. However, the market was sensitive to cyclical fluctuations, which sometimes caused financial problems at the fiber corporations. In 1967, the U.S. market collapsed. In 1970, the European fiber market was in crisis too. The recession that followed the oil crisis of 1973 put the fiber industry in the doldrums. The situation, especially in Western Europe, was dramatic. Many plants were closed down and some traditional fiber producers completely terminated their business.
In the 1960s, high-strength/high-modulus (HS/ HM) carbon fibers were under development at various laboratories. These fibers were aimed at making composites. Carbon fibers were very good but brittle and expensive. They were therefore only used for military applications and in the aerospace industry. At several laboratories, it was found that HS/HM fibers could be made using aromatic or heterocyclic polyamides.
In 1964, DuPont made a fiber that had an unusually high strength and modulus. Soon it was recognized that these higher strengths could possibly be used to make improved tire cords and a cheaper alternative for carbon fiber. In February 1970, DuPont announced its HS/HM fiber in public. The fiber was called Kevlar®.
Other corporations had started researching HS/HM fibers too. Monsanto, Bayer and ICI made several contributions to the technology. AKZO, which improved DuPont’s production process, got into an enormous patent litigation case with DuPont in the 1980s when it introduced Twaron®, a similar fiber to Kevlar. The Japanese company Teijin also developed an HS/HM fiber Technora®. In the Soviet Union, an HS/HM fiber was developed further for military use.
Kevlar was not as successful as DuPont had hoped. It was only used as a tire cord in very specialized tires. The U.S. tire cord market was gradually conquered by the steel wire reinforced radial tire, which had previously conquered Europe. New applications of Kevlar were ballistic protection, composites and replacement of asbestos.
In the 1980s, some corporations carried out research and development to develop fibers of even higher modulus and strength than Kevlar. Stanford Research Institute synthesized two polymers, PBO (poly-para-phenylene-benzo-bis-oxazole) and PBT (poly-para-phenylene-benzo-bis-thiazole), which were further developed by Dow Chemical and Toyobo. Akzo Nobel developed a similar fiber called M5®.
The polymers that were used for HS/HM fibers had a rigid rod structure. However, one also succeeded in making HS/HM fibers from flexible polymers: In 1979, scientists of the Dutch corporation DSM succeeded in spinning HS/HM fibers from a polyethylene gel and stretching it. By this process, they created an ordered structure in the polymers. This polyethylene fiber was in some applications a competitor for HS/HM aramid fibers because it was even stronger. Due to its low melting point it could not compete with aramid fibers in applications in which higher temperatures played a role.
Date added: 2023-10-27; views: 788;
