EDTA & Surfactants. Detailed Description
EDTA or Ethylenediaminetetraacetic acid (also called Ethylenediaminetetraacetate) is a small molecule made of carbon, nitrogen, oxygen, and hydrogen that is really good at binding to positively charged metal ions like calcium (Ca2+), magnesium (Mg2+), zinc (Zn2+) and even iron (Fe2+/3+). It does this by sandwiching the metal ion between its four “arms” very tightly. Have a look at the periodic table at the end of the book and try to find these chemical elements!
During regular cell operation, DNA is bound to cellular molecules called proteins, with the help of metal ions. The proteins are essential for cell operation as they read and copy the DNA. Our goal in this experiment is to get pure DNA. Therefore, we have to remove the proteins bound to the DNA.
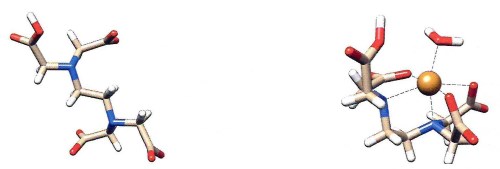
Figure 1-5. EDTA molecule before (left) and after (right) it binds up a metal ion (orange sphere). Dashed lines indicate the bonding between the metal ion and EDTA. By binding most metal ions, proteins no longer bind to DNA. The small red and white V-shaped molecule on the right that is also bound to the metal ion is a water molecule
The proteins cannot easily bind to the DNA on their own - they have help from positively charged metal ions like magnesium (Mg2+) and calcium (Ca2+). The Mg2+ and Ca2+ act as a glue which binds to the proteins and the negatively charged DNA (Figure 1-6). If we can remove the metal ions, then we remove the proteins!
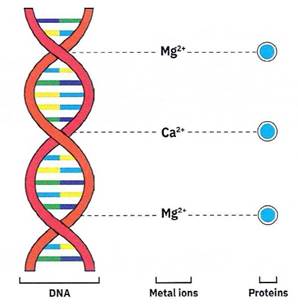
Figure 1-6. Metal ions like a ‘glue’ holding the (-) charge DNA and proteins together
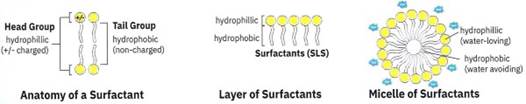
Figure 1-6. The surfactant “SLS” has a charged head that can interact with watery environments and a non-charged tail which doesn’t like to interact with watery environments and other charged heads but like to interact with other non-changed molecules. This results in the surfactant molecule heads interacting with one another and the normal watery cell environment, and the tails interact with each other. Ultimately they form a micelle, a spherical ball with the inside being the surfactant tails and the surface being the charged head group
EDTA is a great way to do this because it binds so tightly to the metal ions. This is also why EDTA is added to shampoo and soaps, it helps to clean you by removing metal ions that bind other molecules to your body. See Figure 4-14 in Chapter 4 to see a similar phenomenon when Ca2+ causes DNA to interact with the surface of cells during genetic engineering.
In addition to EDTA, shampoos and hand soaps have surfactants inside. Surfactants are the molecules that cause bubbles to form. A common surfactant is called sodium laureth sulfate (SLS). Surfactant molecules can bind to many different molecules and sequester them.
They have a hydrophobic end (“water-avoiding”) that can bind to hydrophobic molecules such as fats and oils, and a hydrophilic (“water-loving”) end that can bind to other hydrophilic molecules such as water, proteins and sugars. A general rule to consider when thinking about how molecules interact is “like binds to like”. In other words, molecules that are water-avoiding will generally ‘like’ to interact with other water-avoiding molecules.
When a surfactant is added to the cells, the surfactant “attacks” and cuts into the membrane of the cells (Figure 1-7; Step 1). As the cell breaks into pieces, different cell parts will interact with the surfactants as they form micelles and get parceled away in the micelles (Figure 1-7; Step 2). The same thing happens when you wash your hair! Dirt, grease, and metal ions are gobbled up into the micelles, but your hair is strong enough to hold up against the cutting power of the surfactant.
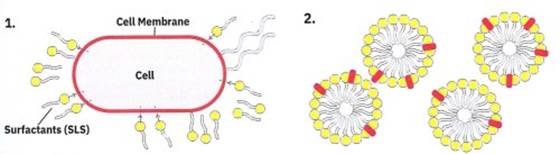
Figure 1-7. (1) The soap molecules called surfactants, attack and cut into the cell membranes of the fruit cells; (2) The surfactants then form spherical jumbles called micelles which contain surfactants and cell debris
Surfactants Pro-tip. There are many different kinds of surfactants used when doing genetic engineering experiments. In this exercise, you used SLS—a common surfactant in body soaps. In laboratories, a very comriion surfactant is called sodium dodecyl sulfate, or, SDS. Another popular surfactant which you will use in later chapters is called Triton X-100.
In general, you can browse the internet to find which surfactant will work for your experiment. In many cases you can use many surfactants for a particular experiment. For example, we could have used Triton X-100 for this exercise. We did not because Triton X-100 is much more expensive and harder to get then body soap, which provides the same result in the fruit DNA extraction.
Date added: 2023-11-02; views: 737;
