Flexible Acclimation to Changes in Light Intensity
The photosynthetic apparatus of higher plants serves two seemingly opposing functions: (a) harvesting of light and transfer of the excitation energy to the reaction centre; and (b) dissipation of excessively absorbed light harmlessly as heat in order to prevent photodestruction of the thylakoids. Not only can the ratio of the antennae to the reaction centres vary but also the dimensions of the antenna complexes can change in response to the light intensity (Fig. 3.8).
The so-called major antenna of PS II, LHC II (light-harvesting complex II, usually organised as trimeric complexes), together with the minor peripheral light-harvesting complexes CP26 and CP29 (chlorophyll proteins, named after their molecular weights in kDa), can dissociate from the core antenna complexes, which are also chlorophyll-containing proteins (CP43 and CP47) surrounding the reaction centre of PS II. When the energy pressure on the reaction centre is high and PS II is overexcited relative to PS I, the so-called state transition occurs (Fig. 3.8). The plastoquinone pool becomes overreduced. This activates protein kinases that phosphorylate threonine residues in the peripheral antenna proteins.

Fig. 3.8. Long- and short-term acclimation of photosynthetic membranes in response to the light environment and to a heat shock. As long-term acclimation under high-light conditions the antennae of photosystem II (PS II) are smaller than those under low-light conditions, thereby reducing light absorption. Short-term regulation: overexcitation of PS II due to excessive light triggers the state transition, a rapid change in the antennae sizes of PS I and PS II. Light-harvesting complex II (LHC II) becomes phosphorylated and partly associates with PS I instead of PS II. (Modified from Anderson and Andersson (1988))
Phosphorylation leads to an accumulation of negative charges and a dissociation of the peripheral antennae from the core antennae. At the same time, the connection of the appressed regions loosens, allowing lateral movement of the peripheral antennae and thus a diminution of the PS II supercomplex (Minagawa 2013). Simultaneously, the extent (as well as the efficiency) of light harvesting of PS I increases, because part of the peripheral antennae of PS II can associate with PS I, thereby balancing the excitation of both photosystems (Fig. 3.9). Such a balance is essential for optimal utilisation of the light energy and is likewise important for the avoidance of PS II overexcitation (over-reduction of the pools of its redox compounds such as QB).
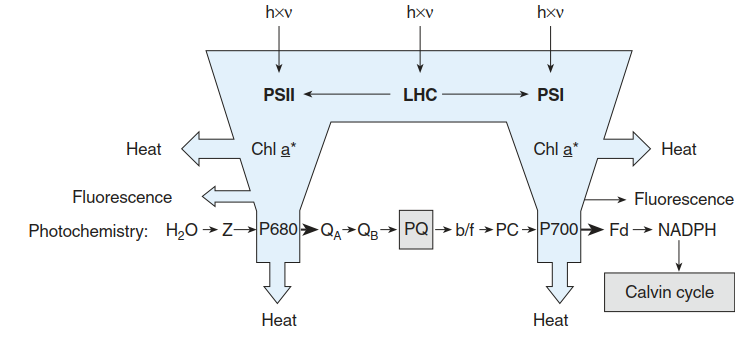
Fig. 3.9. Linear photosynthetic electron transport (from water to nicotinamide adenine dinucleotide phosphate (NADP+)) in the context of utilisation of absorbed energy, and dissipation of excess energy as fluorescence and heat. Acclimation of the photosynthetic machinery to high excitation by the so-called state I-state II transition (dissociation of the light-harvesting complex (LHC) from photosystem II (PS II)) is also shown. The majority of the absorbed light energy is dissipated as heat, and a small proportion (<5%) is emitted as fluorescence. A variable proportion can be used for photochemical work (oxidation of water and electron transport).
Under strong illumination the peripheral antennae of PS II can dissociate from the photosystem and at least in part associate with PS I (state transition; Fig. 3.8). In this way, PS II absorbs less and PS I absorbs more light energy. Since the entire system is dynamic, the rate constants (thickness of arrows) can change—for example, by overexcitation. Photochemistry: presented are the constituents of the linear electron transport (After Schreiber et al. (1994))
Even in the balanced electron flow, light intensity frequently exceeds the capacity of its utilisation for photosynthesis. According to theoretical considerations, 8 mole quanta are required for assimilation of 1 mole of CO2. However, because of the way in which ATP synthesis is coupled to the linear electron flow, slightly more quanta are necessary. The highest measured quantum efficiency (lowest quantum requirement) is 9.4 mole quanta per mole of assimilated CO2. For such measurements, the light intensity must be limiting, as is the case in the linear range of the light response curve (Fig. 3.10, curve a).
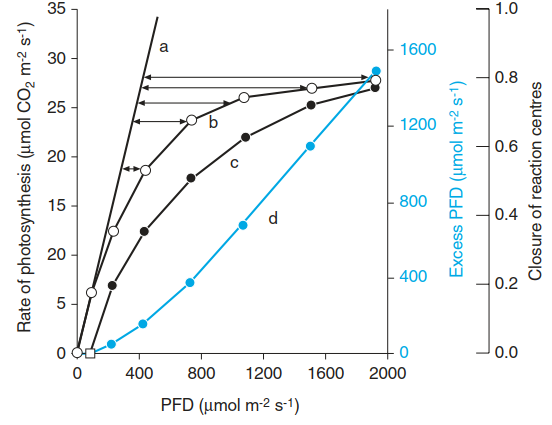
Fig. 3.10. Interpretation of a light response curve of photosynthetic CO2 uptake. Curve a: linear increase of the photosynthetic rate in low light (limitation of photosynthesis by light intensity). Curve b: measured photosynthetic rate indicating light saturation at high photon flux density (PFD). Curve c: calculated proportion of reduced (closed) photosystem II (PS II) reaction centres with excessive light. Curve d: Excess light energy (corresponding to the horizontal arrows between curves a and b). (Modified from Bjorkman and Demming-Adams (1994))
When the light response curve of CO2 uptake deviates from the linear relationship, more light is absorbed than can be used for photosynthesis and the plant is confronted with the need for energy dissipation. At the light intensity of a sunny day (800-1000 pmol quanta m-2 s-1), already 500-600 pmol quanta m-2 s-1 are in excess. The problem of de-energisation of the photosynthetic membranes is aggravated when water shortage necessitates closure of stomata and concomitant lowering of the intercellular CO2 concentration. Similarly, on a bright day in winter, stress arises when the low ambient temperatures greatly decelerate metabolism and metabolite fluxes while high radiation intensities impinge on the leaves. Plants have evolved several mechanisms to cope with this challenge.
In Fig. 3.10, curve d shows the excessive photon flux density that needs to be dissipated. Less than 5% of the absorbed energy can be dissipated as chlorophyll a fluorescence (Fig. 3.9), which is complementary to the portion that can be utilised for photochemistry (photosynthesis).
Because of this relation, photosynthesis can be followed indirectly by measurement of chlorophyll a fluorescence. This is illustrated in Fig. 3.11.
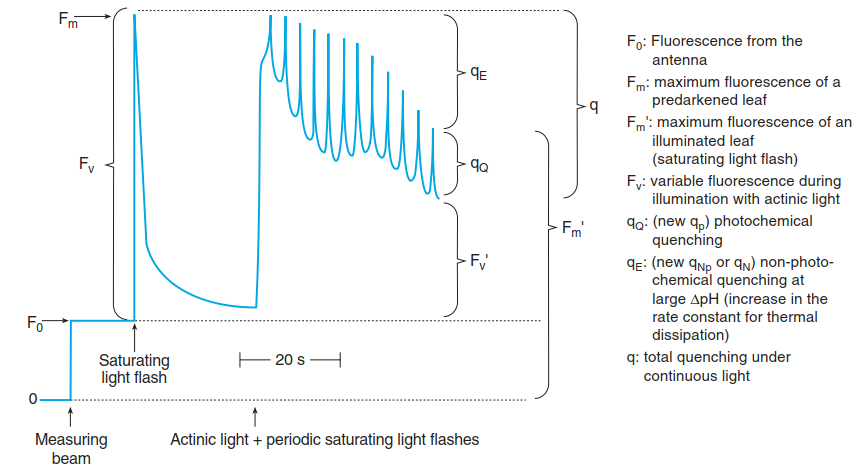
Fig. 3.11. Analysis of chlorophyll a fluorescence from photosystem II (PS II) of a pre-darkened leaf. The first peak reflects the time course of chlorophyll a fluorescence intensity triggered by a single saturating flash on a pre-darkened leaf. A series of saturating flashes results in a decrease of fluorescence by two mechanisms: qP is the quench of fluorescence by photosynthetic electron flow and reoxidation of the reaction centres, while qE reflects a quench resulting from an increasing rate of energy dissipation in the antennae of PS II. F0 is understood to result from fluorescence of some antenna chlorophylls, while FV is the fluorescence that is complementary to photosynthetic electron flow. Actinic light (low intensity) is necessary to maintain the photosystems in an activated state. (After Schreiber et al. (1986))
The intensity of fluorescence is composed of a small contribution from the antennae (termed F0) and a major component from chlorophyll a of the reaction centre. The latter is termed variable fluorescence (FV). When the photosystem is completely reduced—that is, when there is no acceptor of electrons available—FV is maximal (Fmax = F0 + FV). A return of FV to the ground state (F0) takes about 30 s. A series of subsequent saturating light flashes decreases Fm to Fm' because of a quench of the fluorescence of the fully reduced (“closed”) reaction centres.
This quench is again composed of several components: qp is the portion of fluorescence that is quenched by photochemistry (i.e. photosynthesis); qE is the so-called non-photochemical quenching (NPQ) or energy-dependent quenching, which depends on the trans-thylakoid pH gradient, on the concentrations of the xanthophyll zeaxanthin and on the antenna-associated protein PsbS. NPQ is the major mechanism of photoprotection. Excess light energy absorbed by the antennae of PS II is thermally dissipated. As fig. 3.11 shows, this quench increases with increasing saturation/reduction of PS II.
The mechanism of NPQ is complicated insofar that the same ensemble of compounds has to mediate antagonistic reactions—namely, feeding of excitation energy to the reaction centre (of PS II) as well as dissipation of excess light energy by de-energisation of excited chlorophyll molecules. Conformational changes by protonation of the involved proteins, especially of PsbS by an acidic thylakoid lumen pH, result in switching from excitation to dissipation mode (Ahn et al. 2008; Correa-Galvis et al. 2016; Fan et al. 2015).
The dissipative conformation is stabilised by zeaxanthin, which is a product of the xanthophyll cycle (Fig. 3.12). In high light and correspondingly low thylakoid lumen pH, violaxanthin de- epoxidase is activated, which catalyses the formation of zeaxanthin from violaxanthin. A model has been proposed that integrates the fast component of NPQ via protonation of PsbS and the slow component, the formation of zeaxanthin by the de-epoxidation of violaxanthin and subsequent association with PsbS (Zaks et al. 2012, Fig. 3.13).
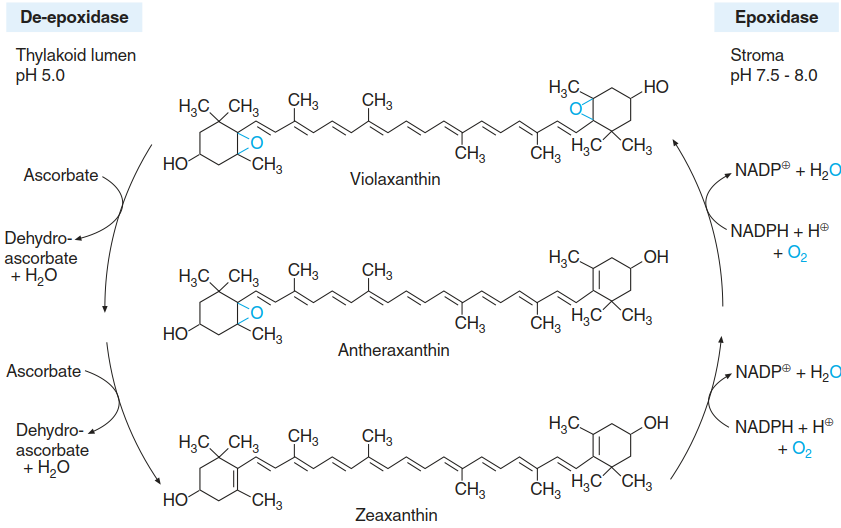
Fig. 3.12. The xanthophyll cycle in chloroplasts. In high light and an acidic luminal pH of the thylakoids, violaxanthin is converted into zeaxanthin by the de-epoxidase; in darkness and a slightly alkaline luminal pH, reoxidation of zeaxanthin to violaxanthin is catalysed by the epoxidase (Heldt and Piechulla 2010)
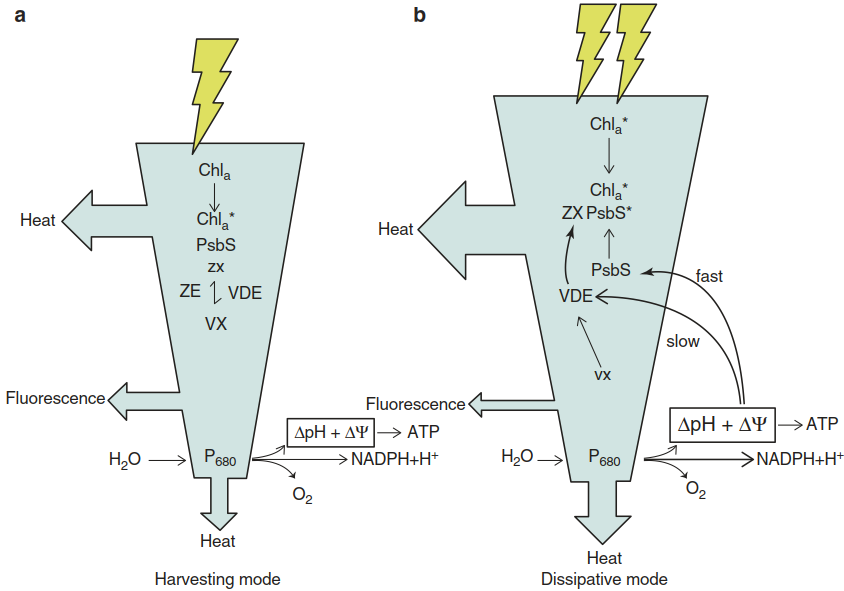
Fig. 3.13. Model for the switching of the photosystem II (PS II) antenna from light harvesting into the energy dissipation mode by non-photochemical quenching (NPQ). a Excitation and flow of energy in PS II under moderate light. The protein PsbS is inactive; the xanthophyll pool consists mainly of violaxanthin (VX) with a very small amount of zeaxanthin (ZX). b Excitation of PS II with high irradiance creates a high proton motive force (ΔpH and ΔΨ). Acidic pH activates (protonates) PsbS, which associates with the antenna, leading to an attenuation of energy transfer to the active centre of PS II and higher dissipation of energy as heat. The Chla*–PsbS* complex is stabilised by zeaxanthin, which is produced from violaxanthin by the pH-activated violaxanthin de-epoxidase (VDE). In low light, NPQ is small or even almost absent and the energy is used for photosynthesis (harvesting mode)
The fast component may be restricted to the already detached antennae, whereas the slow component appears to happen in the core antennae, including the minor chlorophyll proteins, and thus protects PS II from overexcitation (Holzwarth et al. 2009). The quantitatively dominant xanthophyll of the thyla- koid membranes, lutein, can react in a way similar to zeaxanthin (Li et al. 2009). A lutein-5,6 epoxide cycle has been reported, which is driven by light-activated violaxanthin de-epoxidase and zeaxanthin epoxidase under low light (Matsubara et al. 2008). The physiological importance of the xanthophyll cycles is underlined by the increase and decrease in the concentrations of their components upon transfer of a plant from low light to high light, and vice versa (Fig. 3.14).
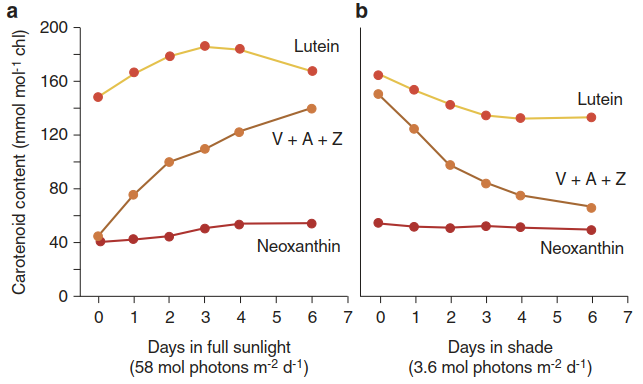
Fig. 3.14. Dynamic changes in xanthophyll pools of young cotton leaves upon changes of the light intensity from low to high a, and vice versa b. In contrast to the xanthophyll neoxanthin, for which no cycle is known, the xanthophylls undergoing de-epoxidation and epoxidation, respectively, respond readily to a change in the light environment. (Modified from Bjorkman and Demming-Adams (1994))
Also, one of the first common garden experiments with A. thaliana wild-type and mutant plants demonstrated that plants with defects in NPQ suffer a significant loss of fitness (determined as seed yield) under naturally fluctuating light conditions (Kuhlheim et al. 2002).
At the metabolic level of the Calvin cycle, even under conditions of closed stomata, a possibility to avoid or at least reduce overexcitation arises from the oxidative photosynthetic carbon cycle (photorespiration) which, under low CO2 (due to decreased conductance of the stomata) and high light, releases CO2 from glycine decarboxylation. This internal CO2 keeps the photosynthetic electron flow running through consumption of NADPH and ATP in the Calvin cycle (for the reactions and compartmentation of the oxidative carbon cycle, see plant biochemistry textbooks).
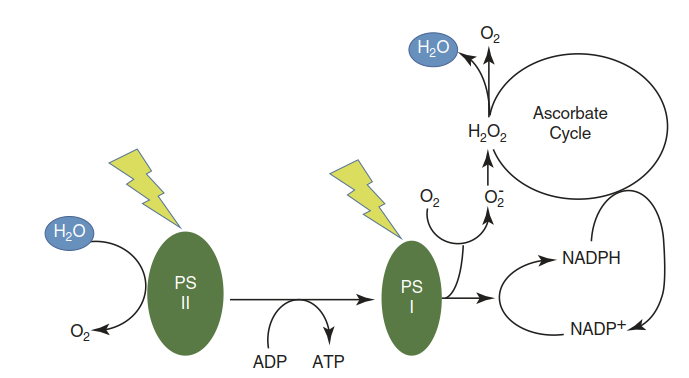
Fig. 3.15. The so-called water-water cycle or pseudocyclic photosynthetic electron transport. This occurs as a recourse of the electron flow when the chloroplastic pools of nicotinamide adenine dinucleotide phosphate (NADP+) and nicotinamide adenine dinucleotide (NAD+) are depleted because of an over-reducing environment and no electron acceptor other than oxygen is available. Reduction of oxygen produces the oxygen anion (superoxide). The oxygen anion dissociates to oxygen and peroxide, which is reduced to oxygen and water by ascorbate peroxidase
Likewise, photosynthetic reduction of nitrite or sulphate and the subsequent formation of amino acids require these photosynthetic primary products. However, the rates of the latter pathways are comparatively small. Oxygen may also be reduced photosyn- thetically in the so-called Mehler reaction, giving rise to ROS, which can be detoxified by a sequence of reactions, finally resulting in the consumption of NADPH (the “water-water cycle” or pseudocyclic photosynthetic electron flow) (Fig. 3.15).
Date added: 2025-01-13; views: 376;
