Parasitic Plants. Descreption
About 1% of all dicot angiosperm species are parasitic. They show a heterotrophic lifestyle— that is, they utilise the photosynthetic products of a host plant by directly feeding on it. The degree of heterotrophy can vary widely and therefore two separate classes of parasitic plants are defined: holoparasites are completely dependent on host photosynthesis, while hemiparasites are photo- synthetically active at least during some stages of their life cycle and sometimes take only water and mineral nutrients from the host (Fig. 8.33).

Fig. 8.33 Parasitic plants. a Hemiparasitic mistletoes on host trees (Photo: copyright obtained from Shutterstock). b The hemiparasite Striga flowering in a maize field
Holoparasites are obligate parasites—that is, they absolutely require a host—while hemiparasites can be either obligate or facultative. The latter type opportunistically exploits neighbouring plants when the opportunity arises. Correspondingly, hemiparasites usually are morphologically indistinguishable from fully autotrophic plants, while some holoparasites lack typical plant structures such as leaves and roots altogether. Plant parasitism, as defined by direct feeding on host plants, is restricted to dicot angiosperms, where it evolved several times independently (Westwood et al. 2010). Commonly distinguished from parasitic plants are myco-heterotrophic plants. They exploit the mycorrhizal connection between another plant and a fungus. In this way they indirectly feed on a host plant. Several hundred plant species have evolved this lifestyle, among them many orchids.
Molecular insight regarding the interaction with host plants is largely restricted to parasitic plants sensu stricto and therefore only these will be discussed further. One reason for this bias is most likely the economic damage caused by parasitic plants such as Orobanche and Striga (Fig. 8.33), predominantly in Africa, where Striga infestation alone affects about 300 million farmers. Consequently, molecular understanding is mostly derived from studies involving species of these two genera.
Parasitic plants can be further divided into root parasites and stem parasites, depending on which host organ they invade. Root parasites, which include Orobanche and Striga species, are more common than shoot parasites (mistletoes are one example). An essential element of parasitism is access to the vascular system of the host in order to draw water, minerals and assimilates. Parasitic plants penetrate host tissues with specialised structures called haustoria. These grow towards the vasculature and establish connections between the parasite and the host (Fig. 8.34). Haustoria are generally seen as the key innovation of parasitic plants and represent the core of the interaction with their hosts (Yoshida et al. 2016). Unlike haustoria of biotrophic fungal pathogens (Fig. 8.9), the haustoria of parasitic plants consist of many cells and remain intercellular in the host—that is, they do not enter host cells.
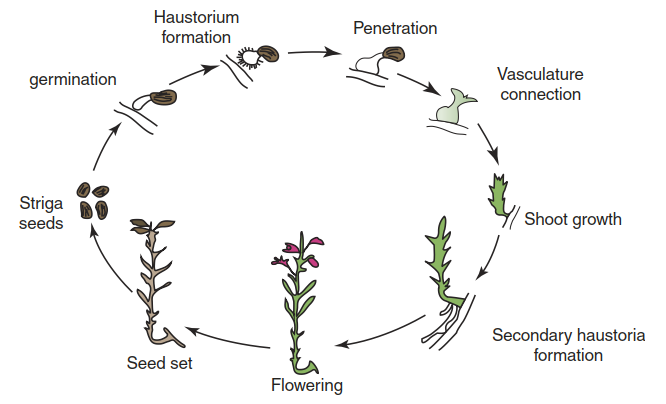
Fig. 8.34. Life cycle of Striga. (Yoshida and Shirasu 2012)
Other critical abilities of parasitic plants, besides haustoria-mediated penetration of host tissue, are the recognition of host plants and the establishment of nutrient transfer from the host. The first step is germination of seeds in response to signals that indicate the vicinity of a suitable host. The seeds of Striga and Orobanche are tiny and thus provide very little reserves for the developing seedlings. Early contact with a host is therefore essential. Striga and Orobanche locate hosts through highly sensitive detection of compounds exuded by the roots of such plants. Many structurally diverse molecules have, over the years, been shown to act as germination stimulants. Those best studied by far are the strigolactones (Fig. 8.35), a class of carotenoid- derived molecules found in small concentrations in root exudates (Fig. 8.35).
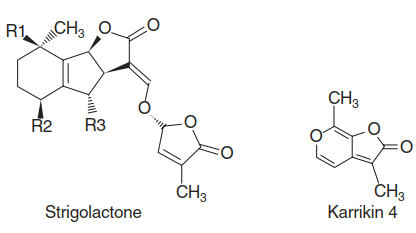
Fig. 8.35. Chemical structures of strigolactones and karrikins
They were isolated as germination stimulants for Striga seeds, hence the name strigolactones (Xie and Yoneyama 2010). For a long time it was not clear why a plant would exude compounds that compromise its performance by attracting enemies. This mystery was solved when strigolactones were first identified as indispensable signals for the interaction with mycorrhizal fungi (Chap. 7) and later as plant hormones that control shoot branching, root architecture and other developmental processes. Perception of strigolactones is thus an excellent example of how parasites or pathogens evolve the ability to use an essential molecule produced by potential hosts as a cue for the presence of such hosts.
Molecules structurally related to strigolactones, so-called karrikins (Fig. 8.35), promote seed germination in a wide range of plant species. Karrikins are found in smoke and serve as fire cues. Vegetation fires provide favourable conditions for seedlings and in many plant species trigger seed germination. Recent findings on the strigolactone receptors of Striga indicate that they evolved independently from the strigolactone receptors ubiquitously present in plants (called D14 in rice and A. thaliana). Ancestral paralogues of D14, the KAI2 genes, mediate the perception of karrikins in many plant species, including species not responsive to fires.
In the parasitic Orobanchaceae lineage that Striga belongs to, the number of KAI2 genes expanded and some of these paralogues represent a distinct clade of KAI2 genes. They perceive strigolactones with very high sensitivity. Their ligand-binding pockets show structural changes when compared with related receptors in non-parasitic plants, meaning that these parasite-specific variants convergently evolved into strigolactone receptors and thus represent one of the molecular components enabling parasitism (Fig. 8.36).
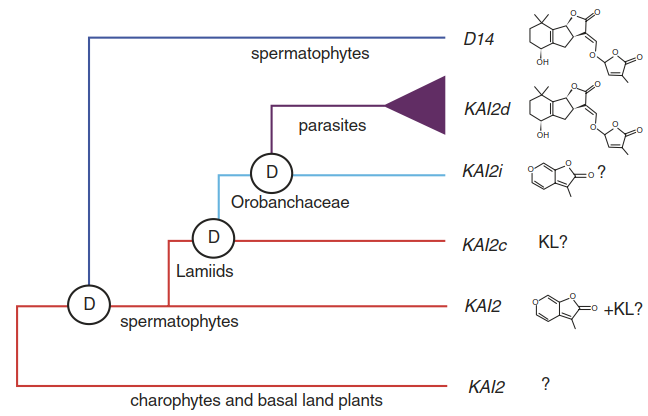
Fig. 8.36. Convergent evolution of strigolactone receptors. Homologues of the KAI2 gene are found in basal lineages such as charophytes. The strigolactone receptor D14 probably arose from these ancestral genes before the evolution of spermatophytes. Additional duplications of related karrikin receptor genes gave rise to rapidly evolving variants in parasitic plants that acquired the ability to detect strigolactones. Thus, strigolactone receptors evolved twice from more ancient karrikin receptors. The second event represents one of the steps towards parasitism. KL KAI2 ligand, D gene duplication. Question marks indicate uncertainty about receptor ligands. (Conn et al. 2015)
Like germination, the next step—haustoria formation—is triggered by chemical signals originating from the prospective host. Again, a range of molecules has been found to show the respective bioactivity, among them flavonoids, quinones and phenolic acids. Perception mechanisms, however, have not been molecularly elucidated yet. Invasion of host tissue is then reliant on localised destruction of host cellular structures by secreted cell wall-degrading enzymes and proteases. Finally, haustoria develop further and connect either with the xylem and phloem (holoparasites) or only with the xylem (some hemiparasites such as Striga). Symplastic connections with host cells enable the acquisition of organic carbon and nitrogen, as well as the exchange of macromolecules (including DNA and RNA) between the parasitic plant and the host plant.
The host ranges of most parasitic plants are rather broad. For example, the majority of Striga species parasitise a variety of grasses. Still, there is some degree of specialisation, as a few Striga species parasitise dicots instead. Also, many other plant species are not colonised, demonstrating the existence of non-host resistance against parasitic plants. The underlying mechanisms, however, are not clear. Penetration of host tissue should elicit wound and defence responses. Whether parasitic plants—in a manner similar to fungal and bacterial pathogens—suppress host defences by effectors that would in turn be recognised by hosts is not clear.
Date added: 2025-02-01; views: 461;
