Methane in Groundwater: Pathways. Methane's Discovery
Introduction. Methane is a ubiquitous trace gas in many groundwater systems, most often a product of in situ anaerobic microbial activity. Many Archaea generate methane via a variety of metabolic reactions that depend on substrate availability. However, in oxic groundwater systems, methane is rapidly oxidized, often to below detection limits. Thus, in the unsaturated (vadose) zone above the water table, there is often no measurable flux of methane to the atmosphere. These oxidation reactions can lead to significant changes in both gas composition and a shift to higher δ13C values for the residual methane. The simultaneous production and degradation of methane in aquifers makes the identification of “fugitive" sources (those not produced in situ; such as from natural gas wells, natural seepage from geologic units, and contaminant spills) difficult. In this article, we will review how methane is formed, modified, and degraded in groundwater aquifers, focusing on a series of case studies that highlight these complexities.
Methane's Discovery. Methane is the simplest of all hydrocarbons, containing one carbon atom and four hydrogens (CH4, or C1). Early human cultures recognized that gases were contained in waters and that some of them were combustible. Piccardi proposed that the famed Oracle of Delphi gained access to her visions from the mix of hydrocarbon gases emitted from seeps. Here, these gases are associated with thick travertine deposits and suggest hydrothermal activity. Methane and carbon dioxide are the major gases produced, and their build-up may have led to hypoxic conditions inside the temple. At other ancient sites, far higher concentrations of methane emanating from seeps and fissures may have led to the “the eternal flame" referenced by Homer. Methane was “officially" discovered by Alessandro Volta circa 1776 who ignited a gas emanating from saturated organic-rich sediments at the shallow edges of Lake Maggiore in Italy.
Risks Associated with the Presence of Methane in Groundwater. Methane is a nontoxic, colorless and odorless gas. Its risk to humans largely occurs when concentrations are high enough to become flammable or, in a confined area, explosive. Methane is reactive with oxygen and thus can lower O2 concentrations in groundwater leading to reducing conditions that affect the overall chemistry of the water. In addition, the presence of methane that is not produced in situ can often be a harbinger of other contaminant issues (e.g. other hydrocarbons and associated fluids).
The past few decades have witnessed the rapid rise of “fracking" (high volume hydraulic fracturing) in many sedimentary basins across the United States, and globally as well. This has prompted research into the assessment of potential gas migration (natural and anthropogenic) into aquifers near sites undergoing active oil and gas development. The leaks that allow methane to enter into groundwater systems may be caused by poor casing of the well upon completion or later degradation of the cement job. However, there is also concern that fracking itself, especially for wells drilled in shallow reservoirs with overlying aquifers, may cause gas and formation fluids to leak into overlying units.
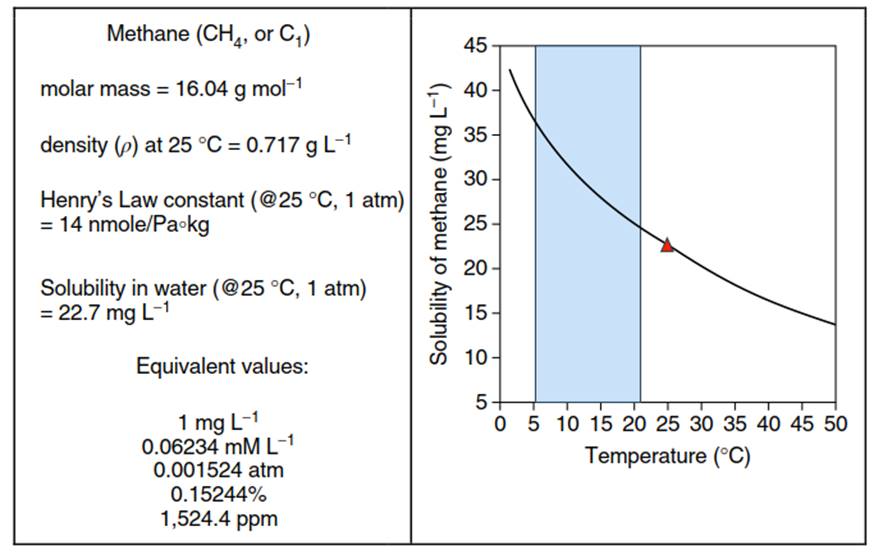
Table 1. Methane constants and conversions and graph of solubility in water at 1 bar atmospheric pressure
The US Department of the Interior actionable level of methane for immediate remediation of a drinking water well is ~28 mgL-1 CH4, although there is reason for concern at an even lower concentrations (-10mgL-1). Care must be taken in reading the available literature as the concentration of methane has been expressed using a variety of different units of measure. The following table presents a series of conversions, gas constant information, and Henry’s Law coefficients. Also shown is the relationship between methane solubility and temperature, which varies significantly over the range commonly experienced in groundwater systems (-5-22 °C) (Table 1).
Date added: 2025-02-13; views: 343;
