Methane in Groundwater: Pathways. Identifying the Source
In Situ or Fugitive? When methane is present above trace levels (~>5 mgL-1) in groundwater, understanding its source is often critical for remediation. Thus far, we have examined the formation and degradation of methane that may occur within an aquifer. These microbial pathways can also occur in underlying formations and the resultant methane can then find its way into the aquifer. In many shallow natural gas reservoirs, microbial gas dominates so that the “fugitive" methane from these sources may be indistinguishable from that microbially generated in the aquifer.
In these cases, the geology, hydrology, and geochemistry of the water in and near the aquifer must be carefully considered. When methane results from the breakdown of residual organic matter (OM) via temperature and pressure (thermogenic gas) or through abiotic reactions (e.g. serpentinization), it is not “sourced" in situ but from far greater depths. We will herein focus on thermogenic gas as the far more likely source for potential groundwater contamination, estimated to make up approximately 80% of our natural gas supply in the US.
Thermogenic gas is produced via the thermal “cracking" of long chained hydrocarbons in the subsurface. At very high temperature conditions (>150 °C), it may occur as nearly pure CH4(often referred to as “dry" gas). At lower temperatures (~70-150 °C) it occurs as a mixture of gaseous hydrocarbons, including methane (C1), ethane (C2), and propane (C3) in varying concentrations (“wet" gas). It is often the detection of the higher chain hydrocarbons (e.g. C2 and C3) that suggests a “fugitive" gas entering the groundwater environment.
It has been widely accepted that compositional (C1/[C2+C3]) and isotopic values from produced gas reservoirs can distinguish (i) thermogenic versus microbial gas, (ii) acetoclastic versus CO2-reduction microbial methanogenesis, and (iii) biogenic versus abiotic methane. However, these simplified geochemical constraints are susceptible to obfuscation due to mixing, migration, and microbial production and degradation.
The increased complexity of gas reservoirs has come to light because of the availability and analysis of the large volume of datasets produced over the past 20 years, in part from the widespread expansion of natural gas from unconventional resources. Unconventional microbial gas from deeper strata migrating upward into groundwater may have compositional and isotopic values that overlap with those of microbial methane that is generated within the aquifer. The canonical Bernard plot, shown in Figure 2 below, should no longer be used as an easy fingerprinting tool to determine the origin of a gas. Indeed, determining the initial source of a gas can be challenging, even if using multiple isotopic tracers (e.g. δD in water and methane; δ13C in carbon dioxide and methane).
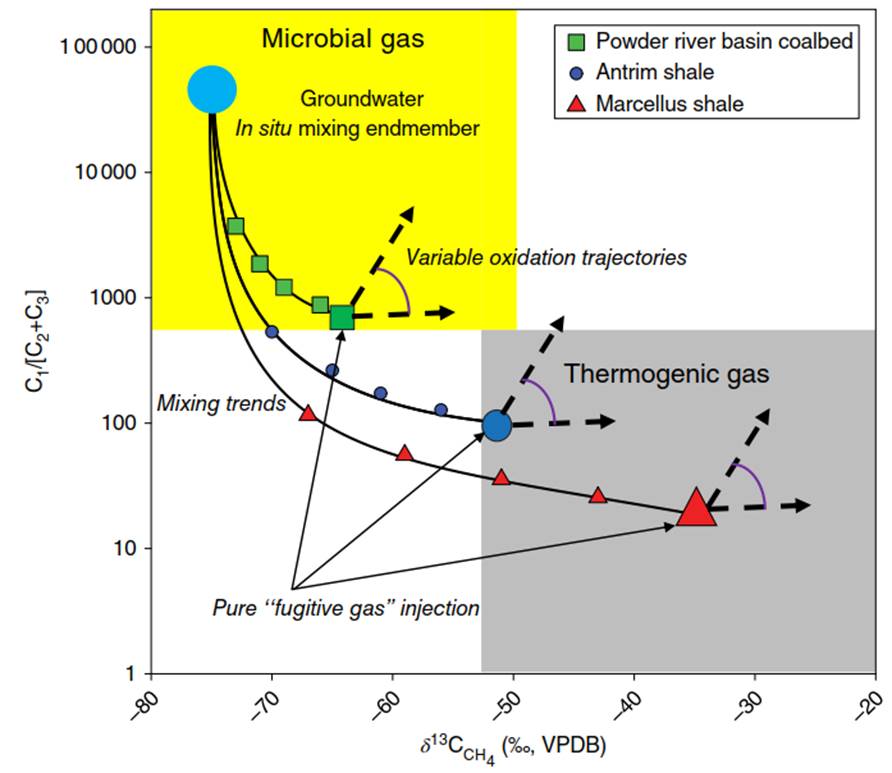
Figure 2. The "Bernard” crossplot of the ratio of methane to higher chain gaseous hydrocarbons versus the isotopic composition of the methane. Fields for thermogenic gas and microbial gas are included, but many processes can affect the original signature of the gas. See text for discussion of mixing and oxidation trends shown
Comparison of the gas composition (C1/[C2+C3]) vs. the carbon isotopic value of the methane has long been used to discriminate thermogenic from microbial sources (Figure 2). Here, we present data from three representative active, hydraulically fractured natural gas reservoirs that could provide a source for “fugitive gas." This gas may enter the aquifer via some conduit, whether natural or induced by drilling, “fracking", or production. The fugitive gas sources range from microbial (Powder River Coalbed - light gray squares), to mixed microbial/thermogenic (Antrim Shale - dark gray triangles), and finally to thermogenic (Marcellus Shale - gray circles). For each endmember, a one-time influx of the gas into a shallow groundwater aquifer is posited.
At the point of injection, each gas retains the isotopic and compositional values of its source (indicated on the figure as a larger symbol). Each of these “fugitive" gases is then mixed with an in situ groundwater methane source (large gray circle). Lines showing mixing ratios are presented and illustrate that when endmembers are known, the influx of relatively little thermogenic gas is quite distinct due to the presence of higher chain hydrocarbons such as ethane (C2) and propane (C3). However, given the variations in composition and isotopic characteristics within these sources, particularly for mixed and microbial gases, there can be multiple plausible blends of sources and subsequent mixing.
Interestingly, while the presence of higher chain hydrocarbons suggests thermogenic gas, their absence does not negate the possibility of a thermogenic source. For example, gas from a very high temperature source rock, having migrated upward in an aquifer (perhaps along a fault), may be classified as a “dry gas," devoid of C2+ hydrocarbons. However, in these cases, it should be identifiable by possessing significantly 13C enriched methane (>-35‰). More difficult to detect is a “wet" thermogenic gas whose ethane and propane have been consumed by methanotrophy, a process that would increase the C1/[C2+C3] ratio so as to make the gas seems more “microbial" from the perspective of compositional ratios.
The C2 + hydrocarbons are degraded by reactions related to those affecting methane (see Eqs. (3)-(5)). In fact, under common groundwater temperature regimes (<25 °C), the higher chain hydrocarbons react more quickly than methane [48]. In all cases, the remaining methane will become enriched with 13Cas the methanogens preferentially oxidize the 12CH4 methane. Arrows describing the range of these paths are shown in Figure 2. Thus, any “fugitive" gas in groundwater aquifers may exhibit isotopic and compositional values that are significantly different from those it possessed when it entered the aquifer due to mixing with other in situ or external sources and through methanotrophic processes.
Summary. The processes that form and degrade methane in groundwater are complicated. The addition of potential external sources (either microbial or thermogenic), with geochemical signatures that may have already been altered prior to finding their way into the aquifer, make it even more complex. A more “holistic" approach than the simplistic “Bernard" plot is required, including a deep understanding of the geologic, hydrologic, microbial, and chemical framework.
Date added: 2025-02-13; views: 337;
