Physical and Chemical Composition of Colloids
Colloids in seawater can be small and globular, composed of humic substances (colored regions in Figure 2), or elongated, fibrillar, and hair-like (white, cotton-like in Figure 2), and other macromolecules/biopolymers. An atomic force microscopy (AFM) picture of such fibrils is shown in Figure 3. Aquagenic fibrillar gel-forming microbial exudates, such as exopolymeric substances (EPS), are classified as “rigid" polymers with Ca2+ stabilizing alpha-helical polysaccharidic regions within exopolymeric fibrils that provide rigidity. These fibrils of EPS are up to several 1000 nm in length, but only 1-3 nm wide, and can exist abundantly attached to cell surfaces or as free colloids.
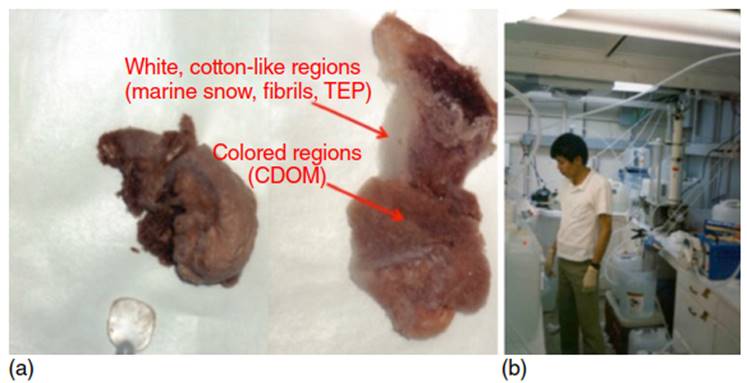
Figure 2. (a) Colloidal samples from Galveston Bay, after desalting and fresh from freeze drying, showing colored (aromatic-rich humics) and white (polysaccharide-rich EPS) regions. (b) Large-volume cross-flow ultrafiltration onboard ship. Source: Photographs with permission by Laodong Guo, University of Wisconsin
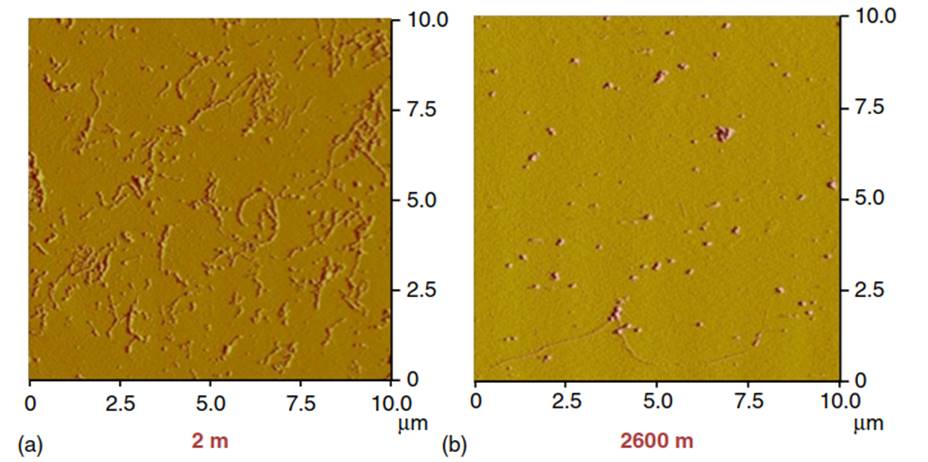
Figure 3. First Atomic Force Microscopy pictures showing forms and shapes of colloids from the Middle Atlantic Bight (2 and 2600 m water depth, showing pearls on necklace, the most common colloidal form, with fibrillar macromolecules 1-2 nm thickness and several microns in lengths) arranged in a "spiderweb” form (a), and mostly small, globular forms with the exception of a close to 10-pm long fibril near the bottom (b). No fibrils were visible at middepth. When these fibrils were subjected to polysaccharide-enriching procedures using alcohol precipitation, pure fibrils showed modern radiocarbon ages, much younger than for the mixture of colloids
While the size and shape of colloids are clearly important, and can constrain molecular weights, information on chemical composition is mainly gained from instrumental techniques applied in organic chemistry. What has been learned in the past several decades from research in marine chemistry, COM is composed mainly of polysaccharides and proteins, but also contains numerous trace elements, radionuclides, and biochemicals such as chl.a, lignin phenols, hydroxamate siderophores, and extracellular DNA.
In addition, marine COM contains pedogenic DOC or geopolymers, which are biologically resistant heteropolycondensations, such as humic-type molecules, and aquagenic DOC that contains products of carbon fixation and consumption, such as EPS that can self-assemble and thus act as glues, and references therein). Both can also act as surfactants or soaps, depending on their relative amphiphilic composition. EPS also have roles in regulating nutrient availability and pollutant sequestration. Both geopolymers and biopolymers contain strong metal-binding ligand and redox-active groups, rendering them as metal-sequestering and redox-state-regulating agents.
Therefore, colloids in aquatic systems can act as vectors of metal removal and of solubilization, depending on the biomolecule and metal ion, as well as modifiers of metal bioavailability. In addition to metal bioavailability in the delicately balanced marine ecosystem, EPS in the marine colloidal pool are also important in their response to global change. The reason is that their production and interactions lead to the formation of sinking material.
In addition, it could also produce cloud condensation nuclei when part of the sea spray. Both sinking particles and sea spray are part of the conveyor belts of global carbon cycling.. Results from such studies indicate that high-molecular-weight COM has relatively high biological and chemical reactivity and short residence times of days to weeks in the water column. Therefore, colloids are important intermediaries in organic carbon cycling in aquatic environments.
Field applications of large-volume cross-flow ultrafiltration also allow one to determine colloidal fractions of trace elements, natural radionuclides used as tracers, and organic pollutants, enabling one to evaluate their removal or dispersive behavior and, in the case of radionuclides, residence times in the water for these colloids. For example, estuarine studies of colloidal associations of selected trace metals demonstrate a spectrum of behaviors across ocean basins such as the Gulf of Mexico. Colloidal associations of trace elements seem to be highest in shallow estuaries such as Galveston Bay, Texas or Ochlockonee Estuary, Florida. In the Mississippi River mixing zone on the continental shelf, colloidal fractions are restricted to the low salinity region. As a consequence of this partitioning, trace elements often partition to colloids similar to the way NOM partitions to colloidal-sized macromolecules.
Date added: 2025-02-13; views: 306;
