Colloids and Nanoparticles in Aquatic Systems
Introduction. Aquatic colloids are nanoparticles and macromolecules in the size range 1 nm to 1 µm with properties that differ fundamentally from bulk solutions. In a true solution containing only ions and small molecules, all the relevant interactions are comparable to the thermal energy, kT. In contrast, a colloidal solution is unstable because of large interfacial energies and because particle-particle interactions have higher interaction energies than kT.
Figure 1 gives an overview of the size spectrum of particles, colloids, nanoparticles, and macromolecules in aquatic systems, along with the major techniques to characterize them. Physical techniques such as diffusion, permeation, light scattering, and viscosity can be used to characterize colloidal systems; chemical techniques can help to unravel their compositional complexities; and biological techniques can give insights into their degradability, bioavailability, and stability. New insights into colloids in marine systems require application of the state-of-the-art techniques that often result in paradigm shifts after successful applications of molecular-level techniques.
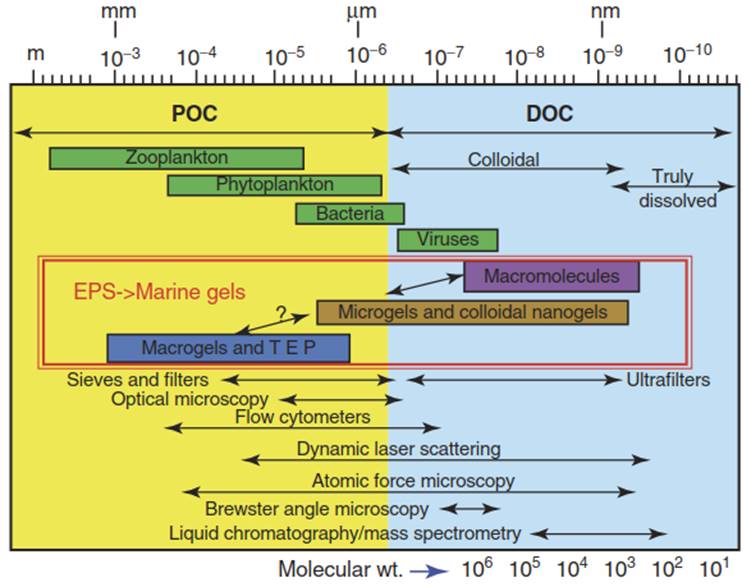
Figure 1. Overview of the size spectrum of particles and colloids in aquatic systems, along with their main characterization methods. Source: Verdugo et al., with permission from Marine Chemistry
Size Separation Techniques for Colloids Sampling. Much of what we know about colloids in the marine environment is through the applications of cross-flow ultrafiltration of large volumes of seawater, an industrial level technique adapted to high salt conditions such as seawater. Ultrafiltration works for both characterization and pollutant-partitioning purposes, and allows to sample large volumes of water. Recent applications of large-volume cross-flow ultrafiltration methods have been reviewed by Guo and Santschi and Doucet et al., and references therein.
These methods have greatly advanced our understanding of the abundance, distribution, physical conformation, chemical composition, and environmental behavior of colloids in aquatic systems, including freshwater, seawater, and groundwater systems. Applications of large-volume cross-flow ultrafiltration systems to sampling marine colloids are now routinely used in marine science (, and references therein). More recently, a newer technique, ultrafiltration coupled to electrodialysis, has occasionally been applied that can sample colloids more quantitatively. This then allowed a more quantitative characterization of the major chemical composition of colloidal-sized macromolecules by NMR.
On the basis of the applications of ultrafiltration, the traditionally defined dissolved organic carbon (DOC) pool, passing a 0.2- or 0.4-µm filter, contains a substantial portion of nanoparticulate colloids, depending on the nominal molecular weight cutoff of ultrafiltration membranes. The >1kDa colloidal organic carbon (COC) comprised up to 40-50% of the total DOC, although this COC percentage can be much higher, up to 60%, in river and estuarine waters.
Colloids can also be size fractionated and characterized simultaneously using flow field-flow fractionation (FlFFF) techniques coupled with online or offline detectors and other separation techniques (e.g. flow cytometry and after appropriate staining of colloids). The coupling of FlFFF with online ultraviolet (UV) absorbance and fluorescence can provide simultaneous separation and characterization of colloidal organic matter, elucidating continuous molecular-size distribution, size-dependent composition, and the heterogeneity of bulk colloidal organic matter (COM) in seawater. The heterogeneous nature of natural organic matter (NOM) in molecular size, composition, and environmental fate can be clearly revealed by the application of the FlFFF technique. Besides FlFFF, dynamic light scattering analysis and flow cytometry [21] have also been shown to give useful information for evaluating equilibrium or dynamic size distributions of colloids and/or gels.
Date added: 2025-02-13; views: 418;
