Adaptations to Flooding-Prone Habitats. Biochemical Modifications
Reoxidation of NADH to NAD+ in fermentative reactions is an absolute requirement to sustain energy provision by glycolysis in low-O2 conditions, since without NAD+ as a substrate, glycolytic reactions cannot take place. Correspondingly, up-regulation of enzymes such as lactate dehydrogenase and alcohol dehydrogenase belongs to the first acclimative responses elucidated molecularly. In flooding-tolerant plants, hypoxia—which under natural flooding stress conditions precedes anoxia—triggers increases in the levels of “anaerobic polypeptides” (ANPs).
This process has been intensively studied in maize roots. Besides fermentative proteins, glycolytic enzymes such as aldolase, enolase and glyceral-dehyde-3-phosphate dehydrogenase have been predominantly identified (Drew 1997). Higher concentrations of these proteins are a combined result of stronger gene expression and preferential translation. The promoters of the anaerobic genes share a consensus sequence, the so-called anaerobic response element (ARE) (Christopher and Good 1996).
The presence of this cis-element allows coordinated regulation. In addition, it has been well documented, at least for alcohol dehydrogenase (ADH) messenger RNA (mRNA), that under hypoxia stress it is more efficiently translated than mRNAs of housekeeping genes. While overall protein synthesis is strongly reduced under low-O2 conditions in spite of continuous gene expression, ADH protein is synthesised at high rates. This is dependent on specific sequence motifs in the 5' and 3' untranslated regions of the ADH mRNA.
Inability to mount these responses is detrimental. Mutant plants with compromised fermentative and glycolytic activities are unable to acclimate to decreasing O2 availability and thus die more rapidly when exposed to anoxia (Bailey-Serres and Voesenek 2008). This has been demonstrated for maize as an example of a flooding-tolerant species. Plants lacking ADH1 are more flooding sensitive than near-isogenic lines with functional ADH1.
An immediate threat of lactate fermentation is a potentially damaging drop in pH (cytosolic acidosis). Therefore, an essential component of metabolic responses to hypoxia and anoxia is the switch to ethanol fermentation. This is brought about by a characteristic of pyruvate decarboxylase—namely, the marked increase in activity at a pH below the usual physiological value. As a consequence, cytosolic acidification due to lactate synthesis switches on ethanol fermentation. Conversely, lactate fermentation is reduced because lactate dehydrogenase becomes progressively less active with the lowering of cytosolic pH. This type of regulation is sometimes referred to as pH-stat (Fig. 5.11). In addition, pyruvate decarboxylase gene expression is induced. An alternative way of counteracting acidification is the activation of lactate efflux in hypoxic root cells. This has been documented in maize.
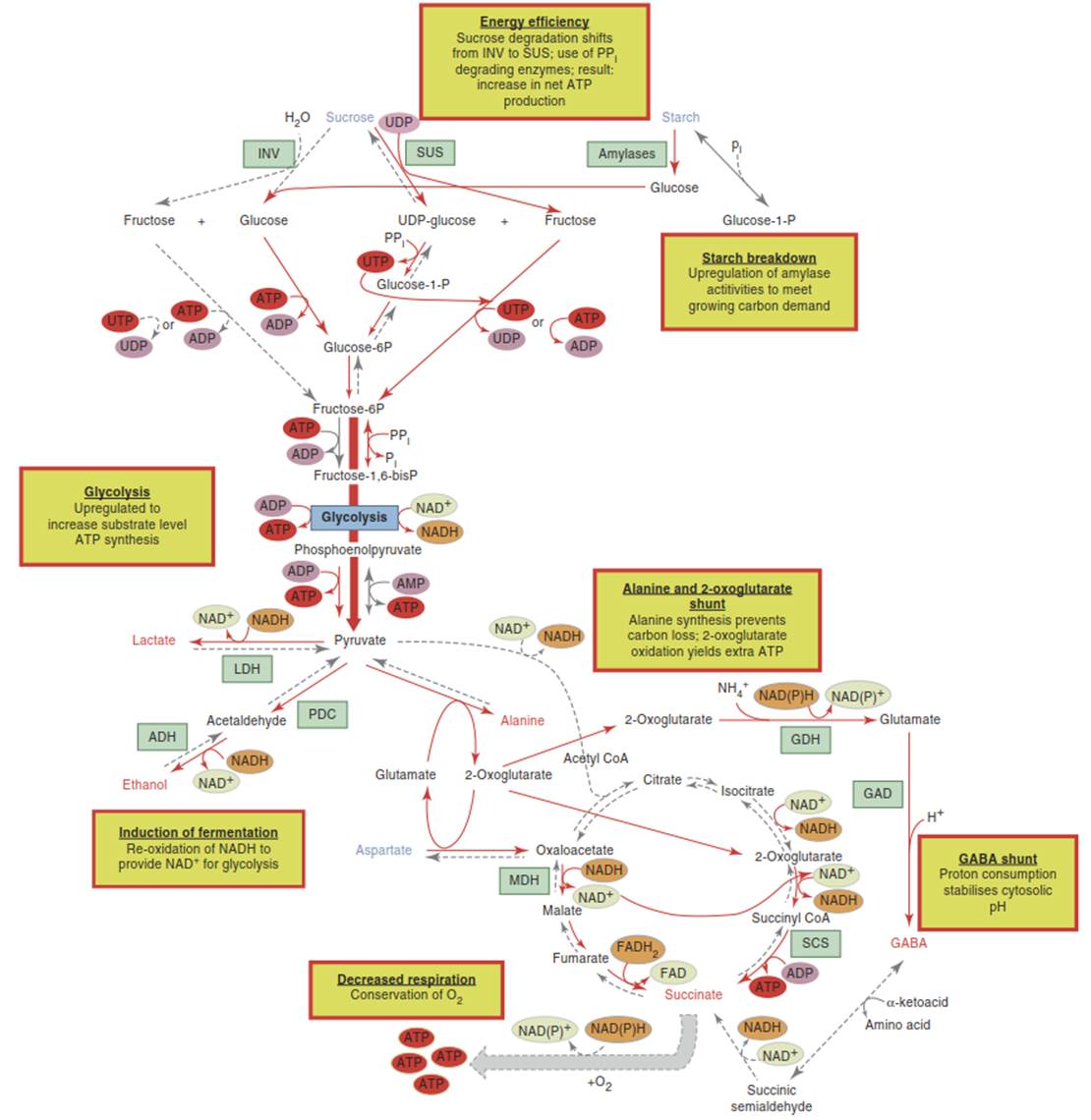
Fig. 5.11. Metabolic adjustments under hypoxic conditions. When respiration is decreased because of reduced oxygen availability, metabolic changes occur that maximise substrate-level adenosine triphosphate (ATP) production and counteract negative consequences of this shift. The processes that are shown have been reported in flooding-tolerant plant species. Please note that they do not necessarily occur all or with the same rates in every species. Sucrose metabolism may be stimulated to meet an increase in carbon demand. Flux through glycolysis is enhanced to at least partially compensate for the much lower ATP yield compared to respiration. Lactate and ethanol fermentation provide nicotinamide adenine dinucleotide (NAD+) to maintain glycolytic activity. Alanine production reduces the carbon loss of ethanol fermentation.
The γ-aminobutyric acid (GABA) shunt consumes protons and thus stabilises cytosolic pH (which can be lowered by lactate fermentation). Yellow boxes summarise prominent metabolic adjustments; red lines indicate pathways enhanced during hypoxia; grey dashed lines indicate reactions that are inhibited during hypoxia. Metabolites that accumulate under these conditions are shown in red; metabolites that decrease in abundance are shown in blue; important enzymes are shown in green boxes. ADH alcohol dehydrogenase, GAD glutamic acid decarboxylase, GDH glutamate dehydrogenase, INV invertase, LDH lactate dehydrogenase, MDH malate dehydrogenase, PDC pyruvate decarboxylase, SCS succinyl CoA ligase, SUS sucrose synthase. (Modified from Bailey-Serres et al. (2012a))
Several additional metabolic responses that support survival of flooding conditions have been discovered in subsets of hypoxia-tolerant plant species (Bailey-Serres et al. 2012a). For instance, more biochemical modifications are known that help prevent some of the negative consequences of fermentation. The loss of carbon due to ethanol formation and its diffusion out of cells is reduced when pyruvate is converted to alanine instead.
The amino group is provided by glutamate, which is converted to α-ketoglutarate. Metabolism of α-ketoglutarate to succinate in the citric acid cycle provides extra ATP. In addition to lactate and ethanol, rice seedlings and sweet flag (Acorus calamus) rhizomes mainly produce basic amino acids (asparagine, arginine and γ-aminobutyric acid (GABA)) as fermentation end products. Synthesis of GABA from glutamate releases CO2 and consumes protons, thereby reducing the risk of cytosolic acidosis (Fig. 5.11).
Reliance solely on glycolysis and the ensuing energy crisis necessitate a suite of modifications to optimise ATP production and to minimise energy consumption. Contributing to the latter is the aforementioned down-regulation of housekeeping protein synthesis. An example of ATP-saving metabolism is a switch in sucrose mobilisation from the invertase pathway to sucrose synthase, which reduces the amount of ATP needed to channel sucrose into glycolysis from 2 moles of ATP per mole of sucrose to 1 mole of pyrophosphate per mole of sucrose (Fig. 5.11).
Because of the low ATP gain of glycolysis in comparison with mitochondrial respiration, energy provision under O2 deficiency is dependent on the rapid mobilisation of starch and other reserves. However, in accordance with the contrasting escape and quiescence strategies, flooded plants differ in the rate of starch mobilisation. Two different metabolic modes of anoxia tolerance can be distinguished, one based on high rates of anaerobic carbohydrate metabolism to supply ATP (energy consumption), and one based on reduced rates of anaerobic carbohydrate metabolism (energy saving) allowing a low rate of energy provision to be sustained for extended periods (Gibbs and Greenway 2003). Over time, many O2-deprived cells move from the first to the second strategy.
Differences in the ability to grow in conditions of primary hypoxia are already apparent at the germination stage and are associated with starch metabolism. Neither wheat nor barley seeds are able to germinate under anaerobic conditions, but rice can because of differences in starch mobilisation (Fig. 5.4). Dry cereal seeds contain reserve carbohydrates, mainly in the form of starch. In order to germinate, they require catabolising enzymes: α- and β-amylase, amylopectin-debranching enzymes and α-glucosidases (maltase, diastase).
In the rice grain, starch debranching enzymes and α-glucosidases are present as inactive precursors, which are activated during germination, even without oxygen. Upon germination in the absence of oxygen, α- and β-amylases are synthesised de novo (Guglielminetti et al. 1995). This happens during the first 2 days of germination when the soluble carbohydrates already present serve as the energy source. After this, starch-catabolising enzymes become active, starch is hydrolytically degraded and the degradation products become available for further metabolism, predominantly as glucose-6-P and fructose-6-P.
An additional aspect of metabolism under hypoxic conditions is post-anoxic stress. Tissues tolerating hypoxic stress are often damaged by subsequent aeration because the sudden availability of oxygen triggers reactive oxygen species (ROS) production. Cells that are metabolically acclimated to hypoxic or anoxic conditions have a comparatively negative redox potential—that is, high electron pressure from a high NADH to NAD+ ratio. In the presence of O2 this leads to oxygen reduction and to the formation of ROS.
During the hypoxic phase, activities of enzymes detoxifying ROS are decreased and the pools of scavenger metabolites are reduced, so the tissue is not capable of coping with increased oxidative stress. However, some plants (e.g. Iris pseudacorus, an ornamental aquatic plant native to Europe, western Asia and northern Africa but invasive in the USA) are known to tolerate post-anoxic stress well, owing to the up-regulation of enzymes such as superoxide dismutase in response to hypoxia.
Date added: 2025-01-18; views: 399;
