Anatomical-Morphological Adaptations and Modifications
An obvious way to alleviate the consequences of inundation with water is the formation of structures that supply oxygen to the roots or, in the case of submergence, to the shoot as well. A hallmark of helophytes (marsh plants) are large intercellular channels extending from the shoot and leaves into the roots. Such gas-filled tissues (termed aerenchyma) maintain a sufficiently high oxygen concentration in the roots.
In plants adapted to conditions of primary hypoxia, the formation of aerenchymas is a constitutive trait. Many other hypoxia-tolerant species (both monocots and dicots) are able to develop aerenchymas in roots and the basal part of the shoot in response to flooding. Documented examples include maize (Fig. 5.7), the coastal grass Spartina patens or sunflower. Formation of aerenchyma not only guarantees the aeration of tissues but also reduces the number of oxygen-consuming cells in those tissues.
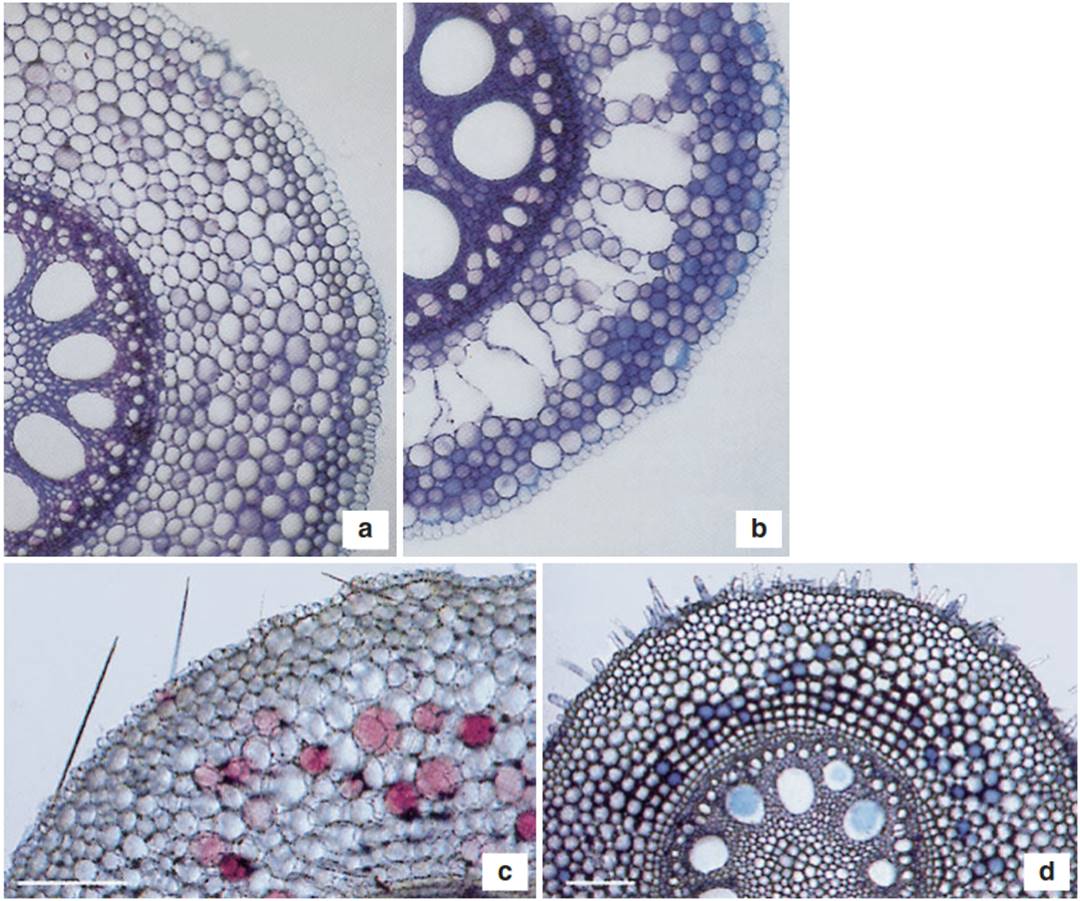
Fig. 5.7. Aerenchyma formation in a maize root: a normoxia; b hypoxia; c hypoxia + neutral red (disintegrating cells are coloured purple by neutral red); d hypoxia + Evans blue (Evans blue accumulates in dying cells). (After He et al. (1996) and Drew et al. (2000))
While, during development, aerenchyma arise by separation of cells at the middle lamella (schizogeny), inducible aerenchyma formation requires programmed cell death (PCD) and disintegration of cells (lysigeny). PCD does not take place in differentiated older cells. Rather, an aerenchyma is initiated near the elongation zone of the organ. The competence to produce aerenchyma has been directly linked to flooding survival. A large-scale investigation of wetland, non-wetland and intermediate species found a clear positive correlation between flooding survival and root porosity (Justin and Armstrong 1987). Such correlations apply also to petioles when partial or complete submergence is considered (Mommer et al. 2006), showing that the formation of longitudinally interconnected pathways for gas flow extends from leaves to root tips.
Another way to enhance oxygen supply is initiation of adventitious roots with a well-developed aerenchyma. Some plants (e.g. maize, ash, willow, Forsythia and Rumex palustris) are able, within a few days, to produce them from basal shoot parts or the lower nodes (Fig. 5.8). These roots do not penetrate as deeply into the soil as the primary root system does into a well-aerated substrate. Formation of adventitious roots involves programmed cell death too. The epidermal cell layer covering adventitious root primordia has to be weakened to allow emergence of the adventitious roots. The mechanical force exerted by the growth stimulation of the primordial cells is sufficient to trigger programmed cell death (Steffens et al. 2012).
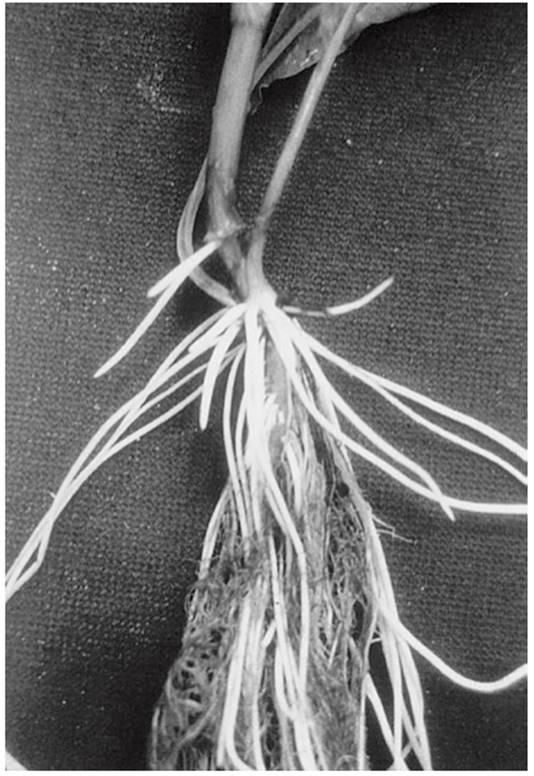
Fig. 5.8. Formation of adventitious roots in the flooding-tolerant Rumex palustris upon flooding of the root bed. The newly formed roots appear white as a consequence of the air-filled spaces in the aerenchyma and are thus clearly distinguished from roots grown under aerobic conditions, which senesce under prolonged hypoxia (Laan et al. 1991)
Oxygen reaching root cells via aerenchyma should be available for respiration and not diffuse out. Radial loss of oxygen from the interior of the root to the surrounding anaerobic soil is often reduced by the formation of a diffusion barrier. This is found, for instance, in deepwater rice, which produces a suberin-impregnated exodermis. Many wetland species show suberised and lignified secondary cell walls very close (within a few millimetres) to the root tip. If such a diffusion barrier tissue is missing, oxygen leaks out of the aerenchyma to the surrounding soil, where the heavy metal ions in the immediate proximity of the roots are oxidised, forming rusty spots and root channels in pseudogley (or stagnosol), the main soil type of wetlands. The oxidation detoxifies the metals for soil organisms—an effect that can also be beneficial for the roots themselves.
Submerged plants additionally show typical leaf modifications that enhance photosynthesis and gas exchange. Leaves of Rumex palustris developing under water have a greater specific leaf area and a thinner cuticle, and the chloroplasts are oriented towards the leaf surface (Voesenek et al. 2006). Complete submergence of plants abolishes access to atmospheric O2. Supply of O2 then is largely dependent on photosynthesis, which explains why light availability can support submergence survival. Some plants, however, show a pronounced “snorkel response” under these conditions—that is, they massively elongate internodes and petioles to escape the hypoxic environment.
This reaction has been termed the low-oxygen escape syndrome (LOES). Applying the definitions used throughout this book (Chap. 2), it would be more appropriately termed an avoidance syndrome, because the consequences (hypoxia) of a stress (submergence) are mitigated by expanding parts of the plant so they reach the surface and have access to oxygen. LOES has been particularly well studied in deepwater rice, where the submerged shoots elongate by up to 25 cm/day (Fig. 5.9).
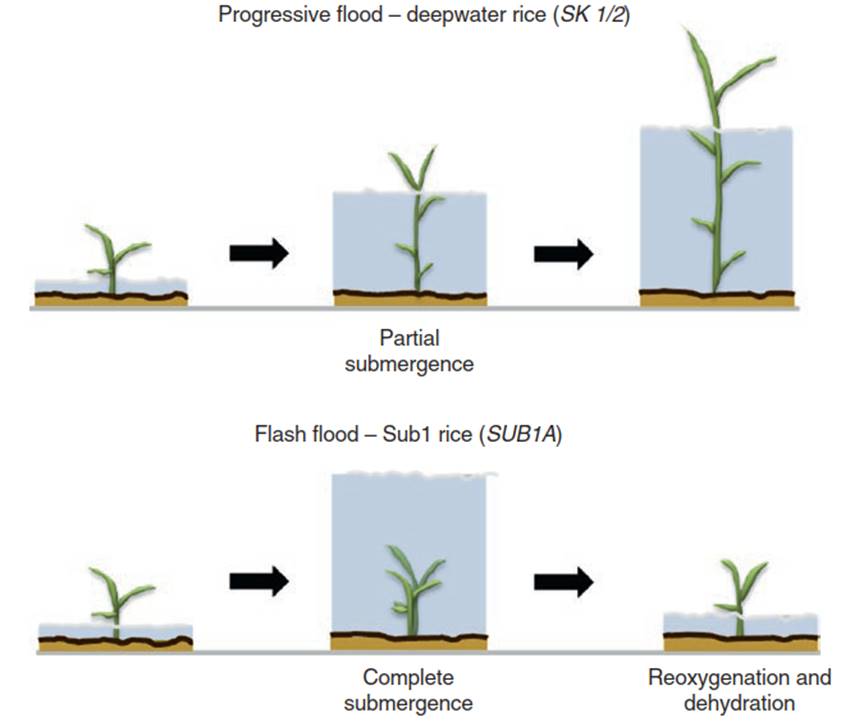
Fig. 5.9. Contrasting flooding survival strategies of rice. Among flooding-tolerant plant species, a continuum of survival strategies can be observed. The extremes of this continuum are represented here by rice accessions. Top: Low-oxygen escape syndrome (LOES); deepwater rice can cope with a slow progressive and long-lasting flood by rapid elongation of internodes. Bottom: Low-oxygen quiescence syndrome (LOQS); some rice accessions endure deep but transient flash floods through a strong reduction of growth and metabolic adjustments that maintain cell viability under anoxic conditions. LOES is controlled by SNORKEL genes (SK1 and SK2); LOQS is controlled by the SUB1A locus. Interestingly, both SK1/2 and SUB1A encode ethylene response factors (ERFs). These ERFs trigger contrasting responses in the respective rice accessions induced by the same signal transduction pathway (Fig. 5.14). (Modified from Bailey-Serres et al. (2012a))
Alternatively, hyponastic growth of leaves (i.e. a change in the orientation of petioles to vertical) can elevate them above the water surface (Voesenek et al. 2006). Such a response is well documented for R. palustris (Fig. 5.10).

Fig. 5.10. Rumex palustris shows hyponastic growth upon submergence. Leaves of a submerged plant (right) re-orient into a more vertical position. Strong petiole growth then moves the leaves towards the water surface. On the left, a plant that was not submerged is shown for comparison (Voesenek et al. 2006)
The strategy to escape low oxygen availability by stimulated growth involves substantial metabolic costs. For instance, cell wall material has to be synthesised, which requires carbohydrates and ATP. Especially given the metabolic constraints that submerged plants are subjected to, these investments can be fatal when the atmosphere is not reached. Indeed, it has been found that the escape is associated with a particular type of flooding—namely, prolonged but rather shallow floods that can be outgrown by internode elongation or hyponastic growth. In the Rhine Valley, elongating species are exclusively found in areas with slow drainage (Voesenek et al. 2004) (Figs. 5.5 and 5.17).
When floods are more transient or deep, escape via accelerated growth as an inducible avoidance strategy is not viable. Instead, species and genotypes exposed to submergence caused by these types of floods display an alternative strategy, the low-oxygen quiescence syndrome (LOQS). They tolerate hypoxic conditions in an energy-saving mode—that is, they restrict growth until the water recedes to a level where above-ground tissues are in direct contact with the atmosphere again. This has been documented best for a limited number of rice accessions (Fig. 5.9) but can also be observed in many other species successfully colonising flooding-prone habitats—for example, other Rumex species such as R. acetosa (Fig. 5.17). LOES and LOQS can be regarded as the extremes of a continuum of survival strategies employed by flooding-tolerant plant species.
Date added: 2025-01-18; views: 405;
