Conditions of Flooded Soil
Common soils consist of four components: soil particles, water, air and organisms (including plant roots) (Fig. 5.1). Freely draining soils can retain water only in pores with diameters smaller than 10-60 µm. Even at water saturation up to the field capacity, the air-filled pore volume is 10-30% of the total soil volume. However, in partially or permanently waterlogged soils, there are almost no air-filled pores, as the air dissolves in the soil water.
Gas exchange in well-aerated soils occurs mainly through diffusion in the continuum of the air-filled pores. It is accelerated by a number of active processes in the soil and thus becomes a relatively fast process. For example, when oxygen is consumed by the respiratory activity of microorganisms and plant roots, oxygen from the atmosphere flows quickly into the soil, following the concentration gradient. As a result, the partial pressure of O2 in the soil air, at least in pore-rich soils, remains in the range of 15-20%. Similarly, CO2 that accumulates in the soil pores quickly leaks out from the soil.
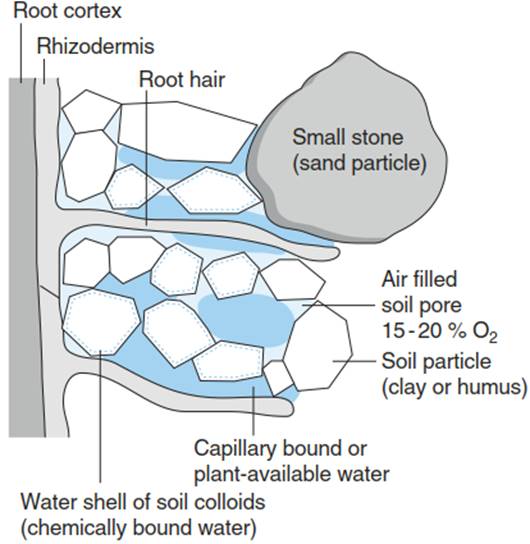
Fig. 5.1. A four-component system: root/soil organism, soil particle, soil water (solution) and soil air
The situation is completely different when gas exchange occurs via the water- filled pores of waterlogged soils. Fick’s first law of diffusion describes the amount of gas diffusing per unit of time (i.e. the net gas flux) as being dependent on the diffusion coefficient, D, the size of the exchange area and the concentration gradient. At the same temperature, the diffusion coefficient of oxygen in water is about 10,000 times (exactly 11,300 times) smaller than in air. Furthermore, oxygen has very low solubility in water (0.03 mL O2 L-1 H2O). Thus, gas exchange in waterlogged soils is very slow and oxygen becomes one of the limiting factors for growth and the development of plants. Similar considerations apply for CO2 supply to submerged photosynthetic tissues. Photosynthesis under such conditions can, in addition, be hampered by low availability of light when the floodwater is turbid.
Long-term waterlogged soils have a negative redox potential because of the low oxygen partial pressure - that is, they exhibit reducing properties. Oxygen entering such soils (e.g. through root or earthworm channels) is readily consumed by soil organisms. The dramatic decrease in the redox potential is already observed after only a few days of flooding (Fig. 5.2).
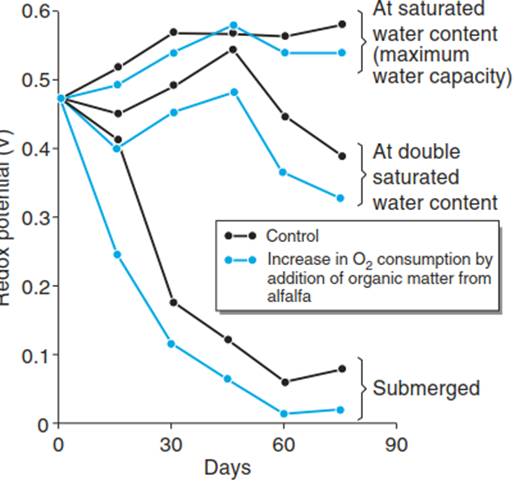
Fig. 5.2. Development of the redox potential of a loamy clay soil as influenced by the water content and the amount of organic matter. (After Amberger (1988))
Microaerophilic and anaerobic microorganisms start to grow. They mainly utilise the organic matter of the soil as an energy source and require ions as electron acceptors. When nitrate is used as an electron acceptor in a process termed nitrate respiration, it is reduced to nitrite, N2O and finally N2 (denitrification). Correspondingly, in sulphate respiration, sulphide is formed from SO42-. These processes reduce the nutrient availability for plants. Similarly, oxidised forms of iron (Fe(III)) and manganese (Mn(IV)) can be reduced to their respective divalent ions. In addition, CO2 may be used as an electron acceptor, resulting in the production of methane. Table 5.1 shows the sequence of redox reactions occurring in the soil when the redox potential decreases. Such reactions often consume pro- tons—that is, they result in alkalinisation of the soil.
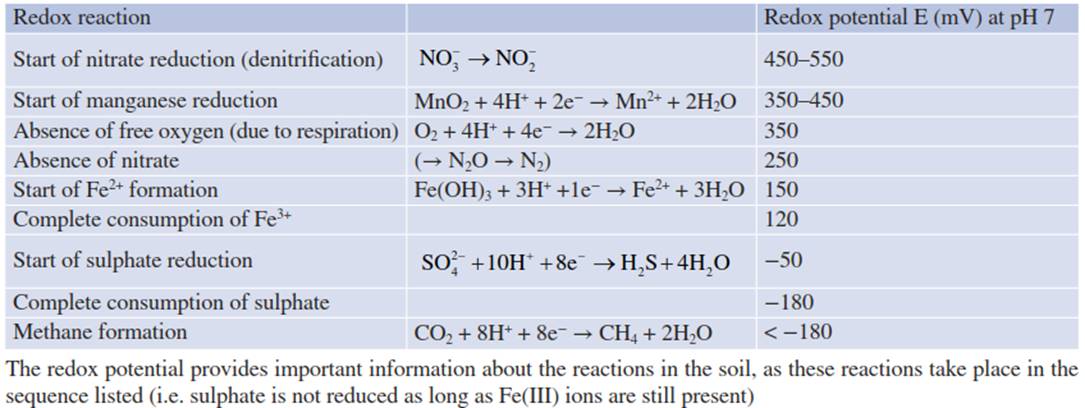
Table 5.1. Sequence of soil-bound redox reactions (After Marschner (1986))
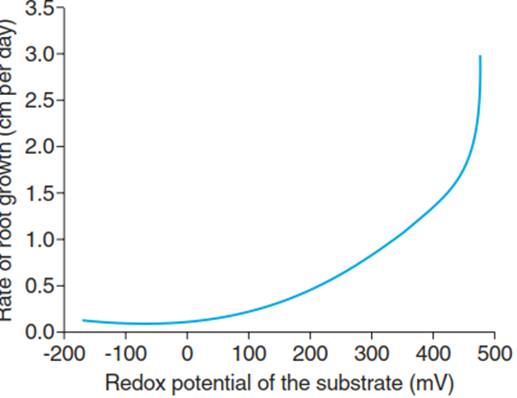
Fig. 5.3. Dependence of root growth of the grass Spartina patens on the redox potential of the soil. (After DeLaune et al. (1993))
Reduced heavy metal ions such as Fe(II) and Mn(II) are more toxic to plants because their availability for uptake is higher relative to the oxidised forms. Thus, the growth of roots not only is inhibited by the lack of oxygen, which is the major stress factor, but can also be affected by toxic ions in the vicinity of the roots (Fig. 5.3). Furthermore, the symbiosis of plant roots with mycorrhizal fungi can be severely compromised in waterlogged soil, thereby further decreasing nutrient acquisition and growth.
Regarding the relationship between oxygen concentration and metabolism, a situation where biochemical reactions are not limited by partial oxygen pressure is called normoxia. When mitochondrial adenosine triphosphate (ATP) synthesis is affected but not completely inhibited by low O2, it operates under hypoxia. In the absence of oxygen (anoxia), oxidative phosphorylation in the mitochondria is negligible and ATP synthesis is restricted to substrate phosphorylation in glycolysis. The necessary reoxidation of reduced nicotinamide adenine dinucleotide (NADH) is achieved by fermentative pathways.
Physiologically, primary and secondary hypoxia (or anaerobiosis) should be differentiated. In primary hypoxia, germination of a plant already takes place in an oxygen-deficient environment, which does not change during the whole lifetime of the plant. This applies, for example, to obligate marsh plants. Secondary hypoxia occurs when plants that normally grow in well-aerated soils are temporarily flooded. This hypoxia gradually develops, allowing plants to acclimate. Accordingly, one has to differentiate constitutively expressed mechanisms to survive prolonged inundation from those induced by flooding events. A second distinction should be made between responses to waterlogging (which affects only root respiration) and submergence (which in addition affects photosynthesis and respiration in the shoot).
Date added: 2025-01-18; views: 379;
