Chaperones and Chaperonins Repair Misfolded Proteins
The HSP70 cycle (Fig. 4.30): The heat shock protein 70 (Hsp70) (DnaK) proteins contain three functional domains; the N-terminal domain has ATPase activity. Hydrolysis of adenosine triphosphate (ATP), as well as the exchange of ATP for adenosine diphosphate (ADP), changes the conformation of the other domains. The C-terminal domain functions as a lid for the interior domain, which binds the misfolded protein(s).
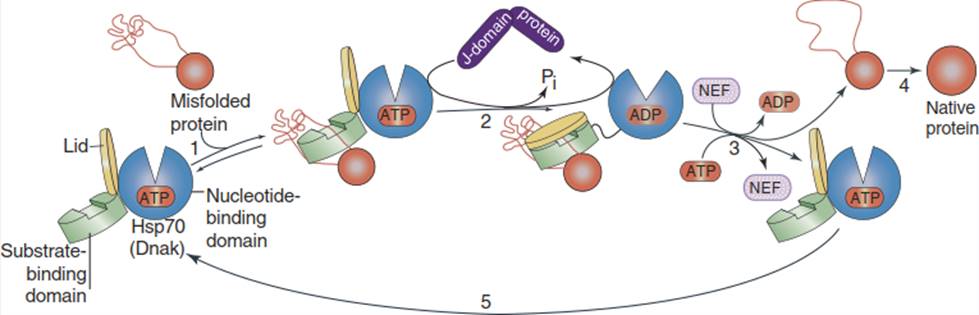
Fig. 4.30. Model of the mechanism of action of heat shock protein 70 (HSP70) chaperones in protein rescue. (Modified from Doyle et al. (2013))
The lid is open whenever ATP is bound to the N-terminal domain. Misfolded proteins bind weakly to the substrate-binding domain by hydrophobic interaction (1). Binding of a co-chaperone (J-domain protein) activates ATP hydrolysis, whereupon the lid closes and refolding of the protein can take place in the groove of the substrate-binding domain (2). The J-domain protein dissociates from the Hsp70-ADP complex.
By interaction with a nucleotide exchange factor (NEF), ADP is replaced by ATP and the lid opens (3) to release the completely (4) or partially repaired protein which, in the latter case, can undergo another HSP cycle (5). Under normal conditions, the HSP70 cycle is also operative in preventing misfolding of hydrophobic stretches of a nascent protein protruding from the ribosome.
The prokaryotic chaperonin GroEL/GroES cycle operates in the plastids and mitochondria of a plant cell in a mechanism that resembles that of the HSP70 system but also shows some differences, as the GroEL forms a back- to-back double chamber, with each chamber consisting of seven GroEL subunits, which all show ATPase activity. Both chambers work alternately. GroES is the lid protein and DnaJ replaces the J-domain protein. One GroEl cycle consumes seven ATPs. Step 3, as the rate-limiting step, requires about 10 s.
Heat Stress Avoidance. The timing of key developmental steps such as germination and sexual reproduction can help plants reduce the probability of heat exposure and damage. Besides that, not many different mechanisms of heat stress avoidance have been demonstrated. At the morphological level, positioning of the leaves in a tree crown or canopy and the leaf angle may reduce heating by sunrays. Segmentation of large leaf areas into narrower parts can have a similar effect. Transpiration cooling appears to be an effective mechanism. However, heat frequently coincides with low water availability, and then the necessary closure of stomata greatly reduces the cooling potential.
More details about heat avoidance are presented in Chap. 9. Generally, however, because of the extreme fluctuations in temperature that can occur daily or seasonally and at the micro-scale, it is in most habitats virtually impossible for terrestrial plants, as sessile organisms, to avoid heat stress altogether. Accordingly, all plants are capable of mounting a response to heat stress (Vierling 1991).
Date added: 2025-01-18; views: 349;
