Ionic permeabilities as integral membrane proteins
In order to utilize the potential energy from the different distribution of ions on either side of the plasma membrane, integral proteins are required. Integral proteins consist of a hydrophobic portion that allows them to be inserted into the membrane and a hydrophilic portion that can form an aqueous pore that crosses the membrane throughout its thickness.
Although water molecules are overall uncharged, they are dipoles due to the presence of a negative charge fraction towards the oxygen atom and a positive charge fraction towards the hydrogen atoms. Therefore, both a positive ion (Figure 4.19A) and a negative ion (Figure 4.19B) in solution are surrounded by hydration water molecules in numbers inversely proportional to their diameters.
An ion in solution can cross the membrane only through the aqueous pores formed by the ion channels and can do so only when hydrated, having a very low probability of crossing the hydrophobic phospholipid layer. These integral membrane proteins are the ion channels which, depending on how they function, can be passive or active.
Aqueous pores as ion channels. These channels are generally open, always allowing ions to pass according to a gradient and are generally present in the cell in limited numbers. Their molecular structure is little-known because they have been little studied. They are inserted into the membrane in such a way as to form an aqueous pore, probably with a diameter and a distribution of charges that select, although not precisely, the ion passing through it.
The flux of potassium and sodium ions through passive channels is small but constant and tends to reduce the potential energy due to the asymmetric distribution of the ions between the inside and outside. The Na+/K+ ATPase, which functions continuously in the presence of ATP and sodium in the cytoplasm and potassium in the external solution, maintains the electrochemical gradients of potassium and sodium that would otherwise be reduced by the passive flows described.
Ion channels. The maintenance of electrochemical gradients is essential to generate the electrical phenomena which, in muscle cells, activate the process of contraction and, in nerve cells, allow the generation and transmission of messages between different regions of an organism and the processing of the messages received in the central nervous system.
These phenomena are temporally confined to a range of a few milliseconds to tens of milliseconds. Macroscopic mechanisms at the cellular level at this order of magnitude require individual elements that activate ion flows across the plasma membrane with much faster kinetics. These elements are the ion channels.
Ion channels are aqueous pores that have a high probability of taking on two conformations from among the infinite number of possibilities: they can be closed or open. Since these are conformational changes of amino acid residues that are part of the aqueous pore, the time to transition between one state and the other is practically null and the probability of the transition depends on changes in the potential difference across the membrane, the time the protein channel remains in that state, environmental factors and specific modulating molecules. All of these factors can alter the probability that the channel protein is in the open state, and therefore permissive to ion passage, or is in the closed state (Chapter 5).
Ion channels are widely used in the mechanisms of excitable cells. Sodium, calcium, potassium and chloride channels produce, control, modulate and re-establish the initial conditions of excitation-related phenomena in nerve and muscle cells. They are, for the most part, voltage-dependent and time-dependent. This means that appropriate changes in the membrane potential and the time the channel is active cause conformational changes in the channel protein, such that the probability of being in one state rather than the other is altered.
Functional, structural and molecular experimental approaches have enabled the intrinsic properties of ion channels to be studied in depth. There are families of sodium, potassium, calcium and chloride channels (Figure 4.20), composed of numerous elements expressed in different organisms or in different tissues of the same organism.
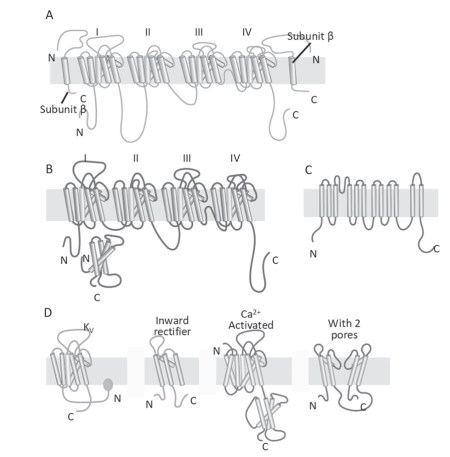
Figure 4.20. Schematic representation of a sodium channel (A), a calcium channel (B), a chloride channel (C) and some members of the potassium channel family (D)
Sometimes channels within a family have different functions, so that each channel must be distinguished with numbers, for example KIR 1.1 or KIR 2.1; the abbreviation identifies the type of channel, the first digit identifies the member of the family and the second the isoform of this member. The sodium, potassium and calcium channel families are encoded by three types of genes that are thought to have evolved from a single ancestral gene, probably a potassium channel with two transmembrane domains (Figure 4.20D); for the chloride channel family, the origin of the encoding genes is not yet clear.
The molecular structure of voltage-dependent and time-dependent ion channels is now known. The basic configuration is that of the potassium channel. This is a homotetramer consisting of four transmembrane domains with six elements each and with hydrophilic amino acid sequences linking the various elements (Kv, Figure 4.20D).
In the case of potassium channels, the gene encodes for a protein that forms one of the four domains. The sodium (Data sheet 4.5) and calcium channels have the same quaternary structure, but unlike the potassium channel, they are heterotetramers. The gene for these channels encodes a protein whose secondary structure comprises four transmembrane domains.
The chloride channel differs from those seen so far. Recently, several models of anion channels have been presented, although there is as yet no definitively agreed structure.
Once activated, sodium channels usually cause an inward current with a depolarizing function. Calcium channels behave in the same way. The latter, in addition to acting on the value of the membrane potential, perform the fundamental function of supplying calcium ions that will be used inside the cell as second messengers (section 3.3).
With regard to repolarization and hyperpolarization, the family of potassium channelsplays an important role. The various potassium channels are used to reestablish rest conditions in excitability processes and modulation of various bioelectric phenomena.
Finally, chloride channels play a key role in modulating nerve excitability and are very important in maintaining the resting potential of skeletal muscle cells.
There are also ion channels in non-excitable cells, mainly calcium, potassium and chloride channels. In these cells, there are also non-specific ion channels, often with mixed selectivity for sodium and potassium. The function of these channels does not depend on voltage or time, but on other factors such as mechanical stress on membranes, cytoplasmic pH, oxidative stress or intracellular calcium concentration.
Voltage- and timeindependent channels are, for example, the sodium and chloride channels of olfactory receptors (Figure 8.2), the potassium and sodium channels of gustatory receptors (Figure 8.4), the sodium channels of the Pacini corpuscle (Figure 8.7), the potassium channels of inner ear Corti cells (Figure 8.13), and the sodium channels of retinal rods and cones (Figure 8.24).
A very important role in non-excitable tissues is played by chloride channels. Chloride permeabilities control cell volume, certain mechanisms involved in cell movement and transepithelial water transport. Point mutations in the protein that forms certain chloride channels are responsible for serious diseases that are still incurable today. A clear example is the APhe508-CFTR mutation causing, among several other mutations, Cystic Fibrosis disease (Imberti et al. doi: 10.1186/s12931-018- 0901-1).
Bibliography: Hille B. Ionic Selectivity, Saturation, and Block in Sodium Channels. A Four-Barrier Model. J Gen Physiol, (1975) 66: 535-560.
Date added: 2024-07-10; views: 526;
