Antifreeze Proteins
In particular, unicellular organisms such as bacteria are endangered by intracellular crystallisation of water. Three strategies have been recognised in frost-tolerant organisms including prokaryotes, lower and higher plants, and special groups of animals—for example, insects or inhabitants of the Arctic and Antarctic Oceans.
Apart from the accumulation of compatible solutes and the activation of their biosynthetic machinery, three functional types of proteins can be differentiated in relation to the formation of ice crystals at the cellular level: proteins that promote ice formation (ice nucleation proteins (INPs)), proteins that inhibit ice nucleation (anti-nucleating proteins (ANPs)) and proteins that control the growth and recrystallisation of ice crystals (the classical antifreeze proteins (AFPs)). ANPs and AFPs are often considered one group of proteins because there is some evidence that AFPs might also bind to nucleation-promoting structures (Griffith and Yaish 2004). Up to now, INPs have been known only from prokaryotes—mainly gram-negative bacteria (Box 4.4)—while ANPs and AFPs are also known from plants.
AFPs have been detected in all types of organ- isms—for example, vertebrates and invertebrates, plants, fungi and prokaryotes (Table 4.9). Their presence can be monitored microscopically by examining the shape of the ice crystals. These are round and flat in water or diluted solutions. Upon binding of AFPs to their prism faces, developing ice crystals acquire a hexagonal structure (Fig. 4.20). AFPs have been extracted from the leaves of frost- hardened plants, but barely from non-acclimated frost-sensitive leaves. For instance, one of the two flowering plants found naturally in Antarctica, Deschampsia antarctica, secretes AFPs into the apoplast (Bravo and Griffith 2005). Thus, the ability to produce AFPs appears to be part of the coldhardening process at least in some plant species.
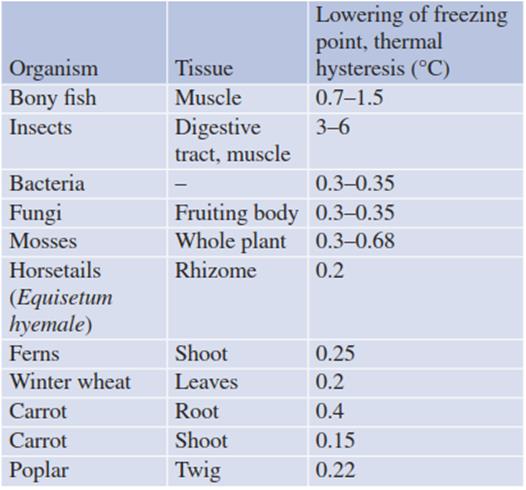
Table 4.9. Lowering of the freezing point by antifreeze proteins, as measured by thermal hysteresis
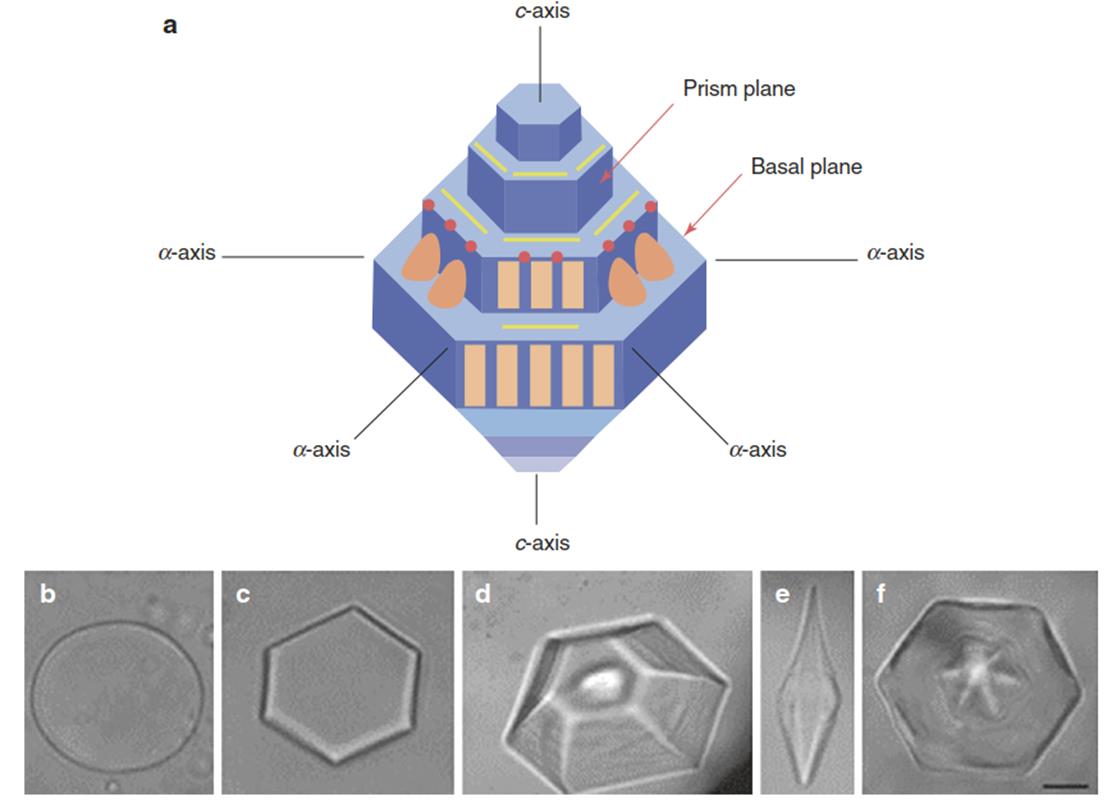
Fig. 4.20. a Hexagonal ice crystal (blue) that forms in the presence of antifreeze proteins (AFPs) showing the a (basal) and c (prism) axes and the modes of interaction with AFPs. Binding of AFPs (brown symbols) to the “vertical” prism plane inhibits growth of the crystal’s basal plane (width), while binding to the basal plane prevents growth along the c axis (crystal length). b In pure water, ice grows as a round and flat crystal. c In a dilute solution, most types of AFP bind to the prism face of ice creating the hexagonal crystals.
d Adsorption of AFPs to the prism face inhibits the binding of additional water molecules, making it energetically favourable for water to bind to the basal plane so that the crystal grows along the c axis (towards the viewer). e At high concentrations of AFPs, the ice crystals form bipyramids a, which are hexagonal in cross-section. f When the temperature is cooled and warmed in slow cycles, it is possible to see ridges on the surface of the ice crystal where the AFPs have bound. Scale bar = 10 µm. (a From Lorv et al. (2014); b-f from Griffith and Yaish (2004))
AFPs are also known as thermal hysteresis proteins (THPs) because they lower the freezing point but not the melting point (thermal hysteresis activity). Thus, the freeze-thaw characteristic appears as a hysteresis curve (Griffith and Yaish 2004) which can be monitored in a temperature-controlled microscope. AFP interaction with tiny ice crystals suppresses their growth, at least at moderate freezing temperatures (ice recrystallisation inhibition (IRI)); considerably deeper supercooling is required for the crystallisation of that portion of water, which corresponds to the equilibrium between the water potentials of ice and the supercooled cellular solutions (Box 4.1).
As they are efficient at very low concentrations (pM), it is clear that AFPs act in a non-colligative (non-physicochemical) manner. AFPs adsorb to the surface of ice crystals by forces that are not yet fully clear; hydrogen bonds, hydrophobic interactions or a particular structure of the hydration layer of the AFPs have been discussed (Lorv et al. 2014). One common character of all AFPs is a flat, hydrophobic ice-binding surface (Smolin and Daggett (2008).
Another common feature are amino acid tandem repeats (X-Gly-Thr-Gly-Asn- Asp-X-U-X-U-Gly-Gly-X-U-X-Gly-X-U-X, in which X = hydrophilic and U = hydrophobic residue), which are aligned on one side forming that surface (Fig. 4.20a).
Adsorption inhibits both further growth of the ice crystal and recrystallisation into harmful shapes, such as long needles. Probably the IRI activity is the more important since the actual effect on the freezing point depression (Table 4.9) is small. In recrystallisation assays with rye leaf extracts, AFPs present in cold-acclimated plants have been demonstrated to inhibit the growth of ice crystals (Fig. 4.20). AFPs are a mixture of small to medium-sized proteins, which can bind to both planes of an ice crystal, covering most of its surfaces. The binding is irreversible until the ice melts.
The efficacy of AFPs in lowering the freezing point differs greatly between organisms. Plant AFPs, although not very effective in lowering the freezing temperature (less than 1 K; Table 4.9) are very efficient in inhibiting recrystallisation of ice nuclei. Because of multiple ice-binding domains of individual plant AFPs or AFP oligomers, the same protein can bind simultaneously to different planes of the ice crystals (Griffith and Yaish 2004). Intracellular AFPs, when binding to ice nucleators, can prevent intracellular ice formation. Furthermore, they can be secreted into the apoplast, where they interfere with growth and recrystallisation of extracellular ice. Thus, they have a dual task.
Amino acid sequences of plant antifreeze proteins do not establish them as a distinct protein family. Instead, AFPs are highly variable and display similarities to a wide range of proteins. Some AFPs—in particular, those from cereals— are highly homologous to pathogenesis-related (PR) proteins produced upon pathogen attack.
They include chitinases, β-1,3-glucanases, polygalacturonase inhibitors, and osmotin- and thaumatin-like enzymes (Griffith and Yaish 2004; Gupta and Deswal 2014)—that is, enzymes that degrade fungal cell walls and inhibit fungal enzymes. In cold-acclimated winter rye, AFPs exhibit both antifungal and antifreeze activity. In contrast, PR proteins induced by pathogens at room temperature in non-acclimated plant lack antifreeze activity. The dual function of the PR-AFPs has been interpreted with respect to resistance against low-temperature pathogens such as snow mould. The excreted PR-AFPs form hetero-oligomers that inhibit growth of such fungal pathogens (Hiilovaara-Teijo et al. 1999).
Date added: 2025-01-17; views: 427;
