Osmotic Adjustments
For the sake of survival during freezing, ice formation should take place outside the cell walls in the intercellular spaces and should be suppressed intracellularly by furnishing of the cells with compatible solutes, which allow them to supercool, thereby preserving their cell plasma in the liquid state. Both strategies entail benefits and stresses or risks. Extracellular freezing of cellular water results in cellular desiccation proportional to the frost temperature. At the same time, intracellular solutions concentrate and may exert ionic stress on biomembranes and protein complexes.
Accumulation of compatible solutes counteracts problems caused by freeze desiccation, but this mechanism carries a high risk as ice formation by overstretching the capacity for supercooling leads to instantaneous death, as there is no time for water export. For both mechanisms, stress increases with the strength of the frost. Possible damage is gradual upon extracellular freezing but is sudden and absolutely detrimental upon intracellular ice formation.
Accordingly, frost hardening of plants is regularly associated with the accumulation of compatible solutes (also termed osmolytes or cryoprotectants) in the cells, predominantly in the vacuoles which, however, have to equilibrate their osmotic potential with the cytoplasm and other cellular organelles. These low molecular weight solutes are carbohydrates, amino acids (e.g. proline), polyamines and many more. Among the carbohydrates that accumulate during frost hardening, sucrose and its galactosides raffinose and stachyose are prominent. Also, polyols, as the reduced forms of monosaccharides, are frequently found. For winter and summer cereals, for instance, a strong correlation between freezing tolerance and the sugar accumulation rate was determined.
Compatible solutes, in addition to their colligative effects, are known also as soft ROS detoxifiers (e.g. mannitol) (Tarczynski et al. 1993). Upon extracellular freezing of cell water, these non-charged but weakly polar (like water) compounds “dilute” the ionic charge at the membrane surfaces (which results from the concentration of cellular ionic compounds) and thus stabilise the bilayer structure.
Their colligative (physicochemical) effect on the freezing point of the cellular liquids comes into play when freeze dehydration progresses. For example, the cell sap of frost-hardened spruce needles is only 1.5 osmolar, corresponding to a freezing point depression of 2.8 K. This is physiologically insignificant because ice nucleation requires stronger supercooling than -2.8 °C. Upon extracellular freezing of cellular water the concentration of the cryoprotectants increases significantly, and with a 10% remaining liquid volume, the freezing point depression attains 28.5 K, which could prevent freezing of the residual liquid water.
An example of the cold-hardening effect of cryoprotectants is the branched-chain polyol hamamelitol and its galactoside clusianose, which accumulate under natural or artificial cold stress in the leaves of the alpine primrose Primula clusiana (Sellmair et al. 1968, 1969) (Fig. 4.19). Both compounds are not degradable by the plant itself and finally reach the soil when leaves decay.
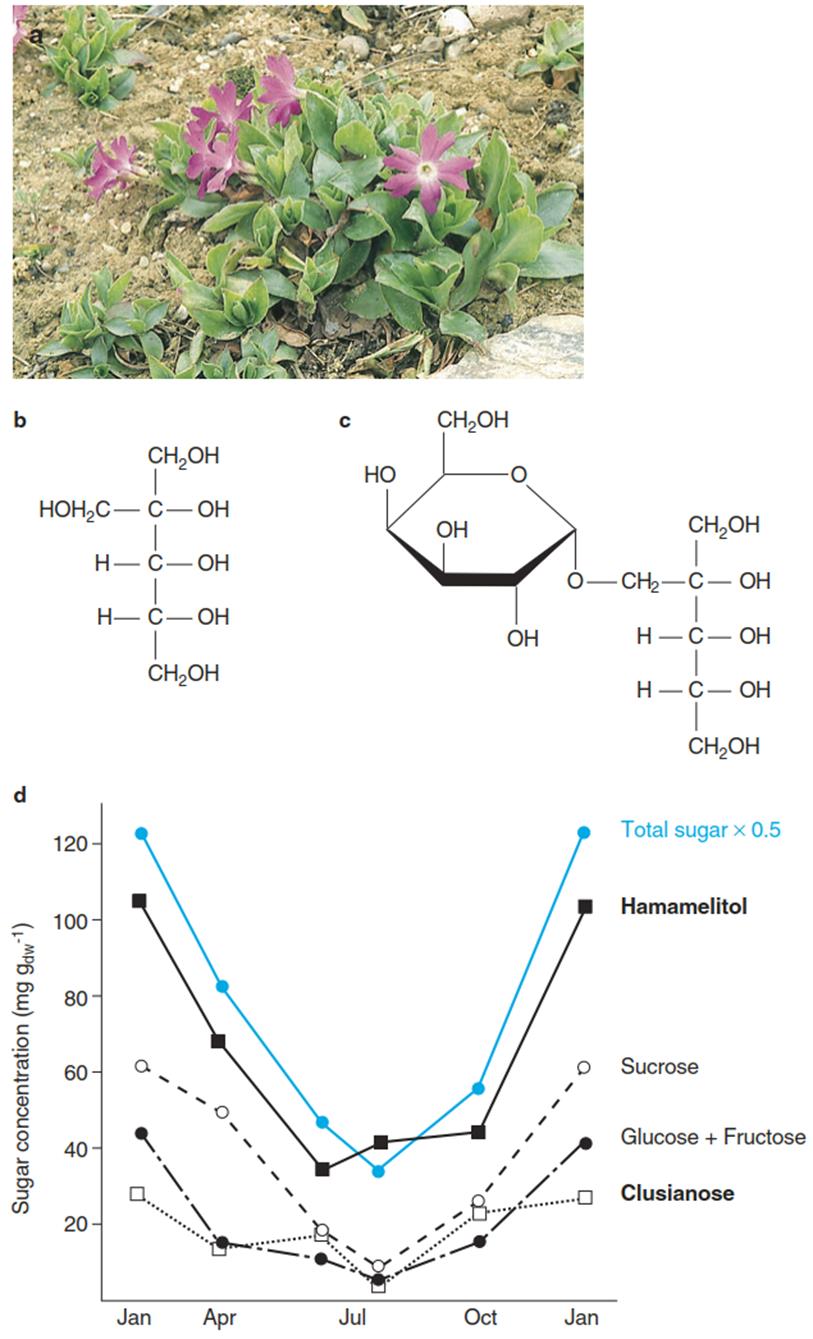
Fig. 4.19. Frost protection in the alpine Primula clusiana Tausch a. b The branched-chain polyol hamamelitol. c The disaccharide clusianose (D-galactosyl-hamamelitol). d Annual course of the concentration of carbohydrates in the leaves of that primrose. Accumulation of carbohydrates can also be induced by cooling of the plants in summer for 1 week at 5 °C. (Photo: E. Beck)
Besides the formation of cryoprotectants, the release of water from the cell into the apoplast can further reduce the probability of intracellular freezing, albeit at the cost of potentially stronger ionic stress for the cell. Membranes of frost-hardened cells allow water to exit from the cell into the apoplast more easily than frost-sensitive membranes do. This has been demonstrated with protoplasts isolated from frost-sensitive and frost-hardened rye leaves (Fig. 4.17). The mechanisms behind this facilitated efflux are not known. The simplest explanation assumes cold inhibition of ion pumps and H+-ATPases, leaking of low molecular weight solutes and passive water efflux, following the solutes.
Date added: 2025-01-17; views: 296;
