Adjustment of Membrane Fluidity
A very important aspect of cold stress physiology is the increase of membrane fluidity with decreasing temperatures. Cold/frost hardening and de-hardening are therefore strongly associated with changes in the chemical composition of the cellular membranes to maintain the fluidity required for proper functioning. Acclimation to a particular temperature involves modification of the lipid composition with respect to the proportions of saturated and unsaturated fatty acids—that is, changes in the desaturation index (double-bond index (DBI)):
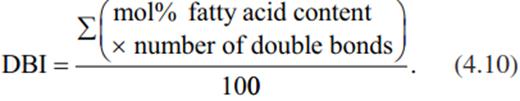
At lower growth temperatures, membrane fluidity is maintained by a higher proportion of unsaturated fatty acids. The respective acclimative responses (Fig. 4.14) have been documented for many different plant species.
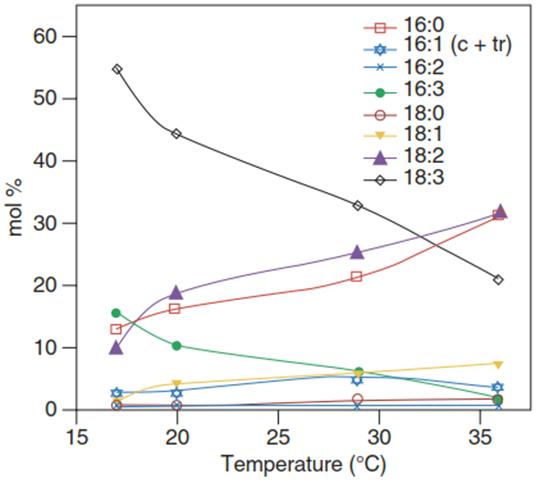
Fig. 4.14. Fatty acid composition of membranes of Arabidopsis thaliana leaves grown at various temperatures from 17 °C to 36 °C. c + tr cis and trans isomers. (Modified from Falcone et al. (2004))
Membrane sterols play a special role, due to an amphipathic structure that differs from that of the glycerolipids. Since their hydrophobic moiety is much larger relative to the polar heads than in glycerolipids, they are able to diffuse rapidly horizontally as well as vertically (the so-called flip-flop mechanism). They serve as buffers for membrane fluidity. At low temperatures, they increase membrane fluidity by disrupting gelling of phospholipid domains and thus preventing the formation of semi-crystalline patches. At high temperatures, they decelerate the motion of the fatty acid tails and thus stiffen the membrane (Buchanan et al. 2015).
Unsaturated fatty acids originate from saturated ones by the oxygen-dependent action of desaturases. Although desaturation is an oxidative process, it requires, in addition, two electrons from electron donors such as ferredoxin (in plastids) or reduced cytochrome b5 (in the endoplasmic reticulum (ER); for more details, see plant physiology and plant biochemistry textbooks). Each fatty acid desaturase introduces a double bond at a specific position—for example, at the ∆6, ∆9 or ∆12 position. In the ER of plants and in cyanobacteria, desaturation is commonly catalysed by acyl-lipid desaturases, which introduce unsaturated bonds into fatty acids that are in a lipid-bound form. Various types of membrane-bound and soluble desaturases are also present in the plastids of plant cells.
The importance of desaturases for cold acclimation is demonstrated by the inability of desaturase mutants to survive at low non-freezing temperatures. In contrast, overexpression of the ω-3 fatty acid desaturases FAD3 (localised in the ER) and FAD7 (localised in the chloroplasts), which catalyse the conversion of linoleic acid (18:2) to linolenic acid (18:3) in tomato, increased the chilling tolerance of the plants (Dominguez et al. 2010) but also affected the photosynthetic performance in a different way, depending on the intracellular localisation of the respective desaturase (Fig. 4.15).
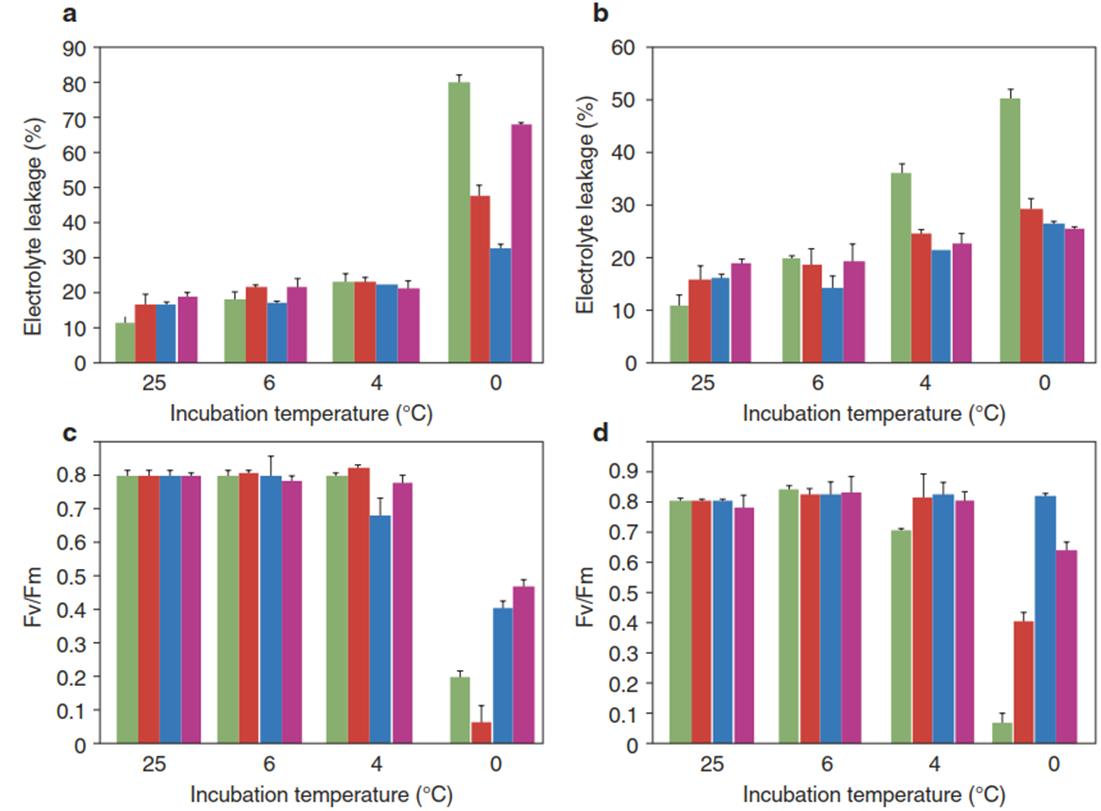
Fig. 4.15. Chilling tolerance and photosynthetic performance of fatty acid desaturase-overexpressing transgenic tomato plants. a, b Chilling tolerance of non-acclimated plants a and acclimated plants b determined with the electrolyte leakage assay. Green bars represent the untransformed wild type; other colours represent independent transgenic lines. Relative conductance was measured before cold treatment and after a 24-h recovery period subsequent to chilling in the dark for 3 days at 6 °C, 4 °C and 0 °C. c, d Photosynthetic performance determined by the chlorophyll a fluorescence assay (Fv/Fm; Chap. 3, Fig. 3.11) of wild-type and fatty acid desaturase-overexpressing tomato before cold treatment (25 °C) and after chilling, as described for a and b. All measurements were taken on the youngest fully expanded leaves. Acclimation was achieved by progressive cooling of the plants to a temperature of 10 °C. (Modified from Dominguez et al. (2010))
Adjustment of the lipid composition of membranes takes time, as degradation, synthesis and trafficking of lipids are required. Temperature changes during the course of a day/night cycle are probably too rapid, as changes in membrane composition usually take several days. Still, during a day/night cycle associated with a corresponding diurnal temperature cycle, the plasma membranes of Arabidopsis seedlings showed slightly lower fluidity during the cold phase. Whether this was due to a reorientation of the membrane domains (van Meer et al. 2008) or to small changes in lipid desaturation was not clear. The fluidity buffer function exerted by the membrane sterols may play an important role in compensating for diurnal fluidity fluctuations (Martiniere et al. 2011).
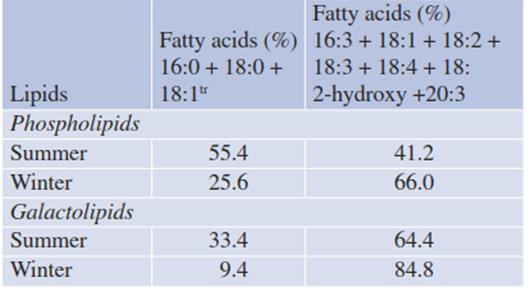
Table 4.5. Changes in the composition of the lipids of a defined biomembrane (the chloroplast envelope of spruce needles) during the course of frost hardening (“winter”) and de-hardening (“summer”) (Senser and Beck 1992)
Evergreen plants of temperate and cold regions are able to seasonally adjust the fluidity of their biomembranes by exchanging certain types of fatty acids for others (homeoviscous or homeophasic acclimation). Such exchanges are a major part of the processes of cold hardening and de-hardening (Vogg et al. 1998). Table 4.5 shows this for a specific membrane—the chloroplast envelope of spruce needles. The proportions of unsaturated and polyunsaturated fatty acids increase dramatically in the phospholipid as well the galactolipid fractions upon cold hardening and decrease upon de-hardening.
Date added: 2025-01-17; views: 367;
