Prevention of Photoinhibition. Cryoprotective Proteins
Cold hardening has to include not only modifications of the lipid/protein composition of the biomembranes but also adjustments of other metabolic processes. The foremost adjustment is the reduction in the light-harvesting capacity of the chloroplasts. The decelerated metabolism in the cold can lead to dramatically increased rates of ROS formation, since light absorption by the photosynthetic pigments takes place irrespective of the temperature, while the biochemical utilisation of the absorbed energy cannot keep pace with the pigment excitation.
Thus, in cold- hardened plants the chlorophyll content is lower and the photosynthetic apparatus is partially degraded (Fig. 4.16). Conversely, xanthophyll concentrations are higher, so the capacity for non-photochemical quenching is higher.
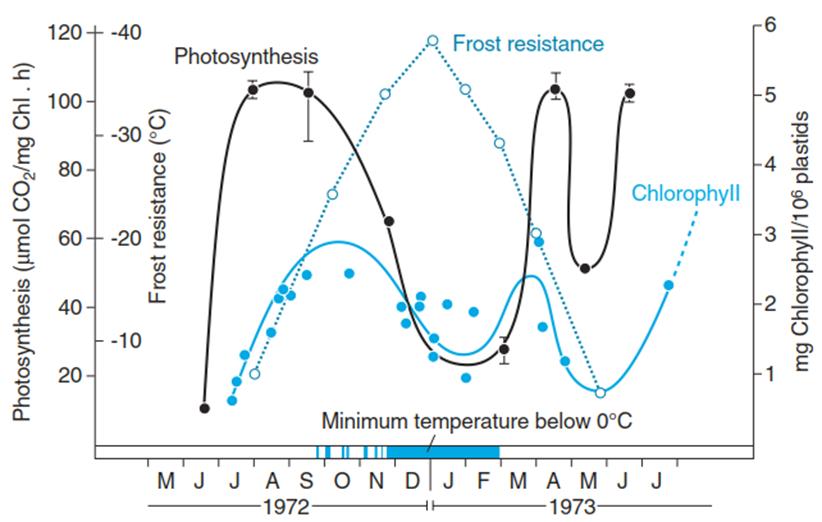
Fig. 4.16. Frost resistance and photosynthetic capacity (CO2 uptake at saturating light and 1% CO2 concentration) of one generation of spruce (Picea abies Karst.) needles measured under identical conditions over the course of 2 years. Note that the annual fluctuation in photosynthesis follows the dynamics of the chlorophyll content of the chloroplasts. Needles were taken from a 50-year-old spruce tree in the Botanical Garden in Munich. (After Senser and Beck (1979))
The limitation of photosynthesis does not necessarily result in a shortage of plant reserve material, as respiration is also markedly restricted at lower temperatures (Hansen and Beck 1994). Concomitantly with the slowdown in photosynthesis, frost hardening of evergreens is associated with a change in carbohydrate metabolism in favour of the production and accumulation of oligosaccharides instead of starch (Hansen et al. 1997).
Cryoprotective Proteins. In the course of cold acclimation, the proteome of plant cells undergoes pronounced changes. Many proteins increase in abundance upon exposure to low temperature. Collectively they are often referred to as cold-responsive proteins (COR proteins). Among them are isoforms of housekeeping enzymes with a lower temperature optimum, as well as enzymes involved in the biosynthesis of cryoprotectants.
The integrity and function of biomembranes have to be protected against damage resulting from freeze dehydration and the concomitant increase in the solute concentrations of the intracellular fluids. Such protection has been associated with the synthesis of dehydrins (DHNs) and dehydrinlike proteins (Late Embryogenesis Abundant (LEA) proteins and Responsive to ABA (RAB) proteins; for more details, Chap. 6). These proteins are all extremely hydrophilic glycine-rich proteins of a wide range of molecular weights.
They lack enzymatic activity, are heat and acid stable and possess a high proportion of a random coil secondary structure, which binds water intramolecularly (Hanin et al. 2011). Interaction with biomembranes is enabled by a hydrophobic domain while the hydrophilic domain(s) face the cytosol. Attachment to the biomembranes displaces low molecular weight solutes, such as ions, from the membrane, thus avoiding changes in the membrane potential and disintegration or inactivation of ion pumps and transporters.
In unstressed plants, only low concentrations of dehydrins are present. Stress by drought, salt and cold leads to rapid up-regulation of gene expression and steady accumulation of dehydrins in all compartments of the cell. As for the majority of the COR proteins, formation of dehydrins can be triggered by abscisic acid (ABA) or can be activated independently of ABA. The promoter of the COR gene, RD29A (also known as COR78 or LT1178) contains cis-acting elements for ABA-independent signalling (cold response, osmotic stress) as well as for ABA-dependent (osmotic) signalling. Enhanced frost tolerance of A. thaliana was achieved by overexpression of RcDHN5 from Rhododendron catawbiense (Peng et al. 2008) and was attributed to maintenance of enzyme activity upon water deficiency. Nonetheless, the question as to whether particular dehydrins directly contribute to chilling and freezing tolerance remains unanswered.
Another type of proteins has been described as Cold Shock Domain Proteins (CSPs), suggesting cryoprotective properties (Sasaki et al. 2007). The typical Cold Shock Domain (CSD) contains a five-stranded β-barrel sheet with two consensus motifs, which bind to single-stranded DNA/RNA (Sasaki et al. 2013). The CSDs are found in bacteria, as well as in eukaryotes, and are considered RNA chaperones. Upon cold treatment, RNA forms unfavourable secondary structures that could interfere with RNA functions such as transcription and translation. CSPs are able to resolve these structures and restore RNA functionality. Although they are associated with cold adaptation, CSPs regulate many biological processes such as growth and flowering, thus exhibiting pleiotropic effects.
Date added: 2025-01-17; views: 365;
