The Heat Shock Response. Heat Stress Transcription Factors
Heat stress causes the unfolding (denaturation) of proteins. In eukaryotic cells this triggers a reaction sequence termed the unfolded protein response (UPR). Stress-induced accumulation of unfolded protein activates transcription factors (heat shock transcription factors (HSFs)), which then bind to specific cis elements (heat shock elements (HSEs)) in the promoters of genes encoding heat shock proteins (HSPs).
Heat Shock Proteins.HSPs are named after their enhanced production upon heat treatment. However, they are essential for prokaryotic as well as eukaryotic cells, both in the absence of stress and for heat stress survival. HSPs act as chaperones required for correct folding of (unglycosylated) nascent proteins upon release from the ribosome or after stress-induced misfolding, unfolding or aggregation of unfolded proteins (Box 4.6). This essentiality is the reason why the contribution of HSPs to heat stress survival—while not in doubt—cannot be accurately assessed genetically. Loss-of-function mutants are not viable.
HSPs are constitutively expressed and involved in protein and membrane stabilisation, in protein assembly into functional complexes, in intracellular transport of protein precursors to their target organelles and as part of cellular signalling processes. In plants they occur in plastids, mitochondria, peroxisomes, the nucleus, the cytosol and the ER. However, not all chaperones occur in all of these plant cell organelles. Prokaryotic HSPs such as HSP60 are known only from mitochondria and chloroplasts—plant organelles of prokaryotic origin.
HSPs are classified by their molecular weight (Wang et al. 2004) and only in the bacterial (Escherichia coli) system do they have specific designations (in brackets). The most important classes are HSP100 (Clp), HSP90, HSP70 (DnaK), HSP60 (GroEL, the so-called GroE chaperonins) and the small HSPs (sHSPs): the HSP40 (DnaJ), HSP10 (GroES) and HSP23 (GroE) families. HSPs can cooperate with co-chaperones (e.g. HSP70 with HSP40 and HSP23).
Chaperones, particularly of the HSP70 type, are also involved in the removal of irreversibly denatured proteins. After a strong heat stress, some unfolded proteins cannot be repaired anymore. They are irreversibly denatured and tend to form—or have already formed—aggregates via their exposed hydrophobic domains. Such proteins have to be removed from the cell. They are tagged for degradation in the 26S proteasome by the attachment of several units of the small protein ubiquitin (poly-ubiq-uitination). Targeting to the proteasome where the adenosine triphosphate (ATP)-consuming degradation takes place is then dependent on HSP activity.
Heat Stress Transcription Factors.Transcriptional activation of HSP genes is dependent on heat stress transcription factors (Hsfs). Hsfs have a modular structure (Box 4.7) which, irrespective of variations in size, is conserved in the entire eukaryotic kingdom. The mode of interaction with the promoters is equally conserved. Plant genomes are particularly rich in Hsfs: in A. thaliana, 21 Hsf genes are known; in soybean there are 52 such genes. According to similarities and differences in their sequences, plant Hsfs have been classified into HsfA, HsfB and HsfC, each class consisting of a number of members (e.g. HsfA1-A8). Further grouping is indicated by lower-case letters (e.g. HsfB4a-h). In comparison with plants the diversity of Hsfs in other organisms is smaller.
Hsfs mediate the acute heat shock response, as well as the acquisition of thermotolerance. Thus, responses of different magnitude can be controlled by Hsfs. This is exemplified by representatives of the A1, A2 and B1 groups in tomato (Fig. 4.31). Under normal conditions (Fig. 4.31a), monomeric HsfA1 is inactive because of its association with HSP70 and HSP90, while HsfB1 is targeted to the 26S pro- teasome for degradation. Acute heat stress and concomitant denaturation of proteins results in the dissociation of the chaperone-Hsf complexes and association of the HSPs with the misfolded or unfolded proteins.
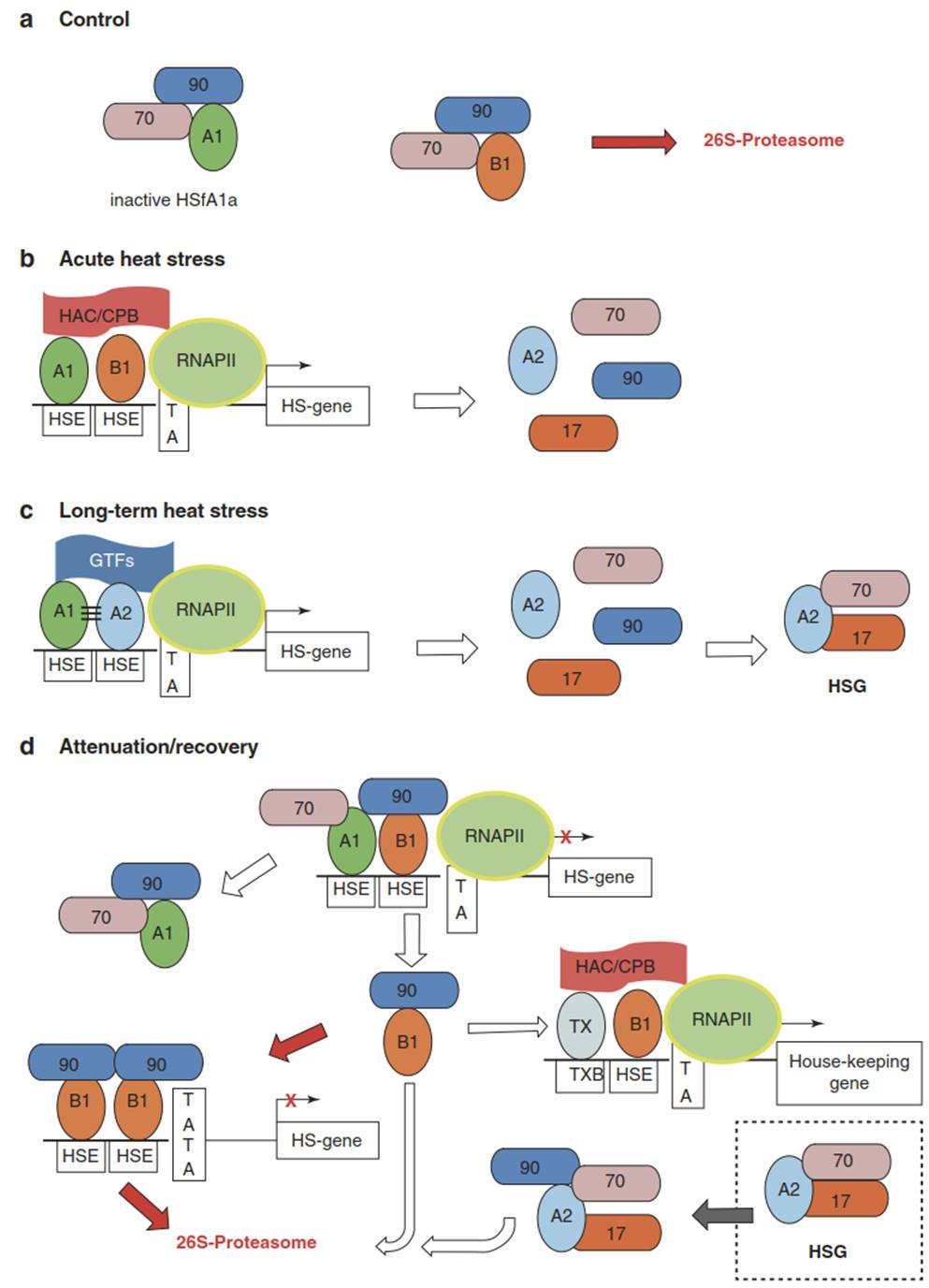
Fig. 4.31. Regulation of the heat stress response in tomato. a The unstressed state. b Acute heat stress; free Hsfs oligomerise and bind to the heat stress elements of the promoters of the HSPs. More HSPs are produced, as well as more HsfA2, the key regulator in the plant heat stress response. c Prolonged heat stress leads to acquired thermotolerance; Hsfs A1 and A2, together with other general transcription factors (GTFs), form a superactivator for the expression of more HSPs and the formation of heat stress granula (HSG). d Recovery from heat shock and enhanced thermotolerance; expression of HSP genes is strongly attenuated and the Hsfs are either degraded in the proteasome or conserved by binding to HSPs. (Modified from Scharf et al. (2012))
The liberated Hsfs (particularly HsfA1 as a master regulator) oligomerise (trimerise), and HsfA1 and HsfB1 bind to the heat stress elements in the promoters of heat stress-activated genes, which usually harbour more than one HSE. The HsfA1-HsfB1 complex further recruits a histone acetyltransferas (HAC1-CBP in Fig. 4.31) as a co-regulator, and this ternary complex enhances the expression of the HSPs and, in particular, that of Hsfs B1 and A2, which play a crucial role in the acquisition of thermotolerance. HsfA2 is expressed only in stressed plants and accumulates to high levels upon long-term heat stress. Loss-of-function and overexpression experiments have revealed HsfA2 as one of the key elements in the plant stress response.
HsfA2 is involved in protection against heat, ROS, salt stress and even anoxia. It hetero-oligomerises with HsfA1, forming a kind of superactivator complex (a so-called enhancosome) for the expression of HS genes (Fig. 4.31c). Apparently, the combination of the HsfA2 activator domains (AHA in Fig. 4.32) with the HAC1-CBP results in strongly enhanced activation of the transcription machinery. Heterooligomerisation with HsfA1a is also necessary for the import of HsfA2 into the nucleus in order to overcome the strong C-terminal nuclear export signal (NES) of this Hsf.
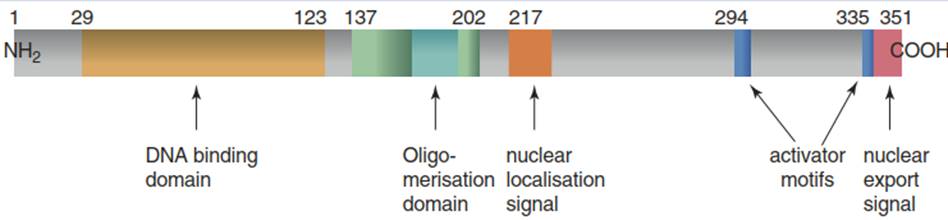
Fig. 4.32. Domains of the tomato HsfA2. (Modified from Scharf et al. (2012))
Upon abatement (Fig. 4.31d) of the heat stress, the heat stress response is switched off. HsfA2 is inactivated by association with small HSPs (e.g. HSP17-CII and HSP70). This complex accumulates in the so-called heat stress granula. Release of HsfA2 from these granula requires another small heat stress protein, HSP17C-1, which is important for longterm heat stress. Prolonged heat stress results in high levels of heat stress proteins and of HsfA2. This is the state of acquired thermotolerance. Fading of the heat stress releases HSPs because of the decreasing level of misfolded proteins.
An increase in the concentrations of free HSPs leads to monomerisation and thus inactivation of the Hsfs because of their reassociation with the HSPs. HsfA1 binds again to HSPs 70 and 90, and HsfB1 binds accordingly to HSP90. This complex can elicit degradation of HsfB1. Furthermore, it can interact with transcription factors of housekeeping genes and reactivate their expression. HSP90 interacts with HSGs, whereupon HsfA2 is released and degraded.
The interaction between heat shock factors and heat shock proteins exerts effective control over the up-regulation of HSP synthesis. The presence of more denatured proteins than under non-stressed conditions triggers the interaction of Hsfs with HSP gene promoters, and a decline in the number of denatured proteins gradually tunes out the extra HSP synthesis. Nonetheless, other factors are known to contribute to the activation of the heat shock/heat stress response (Kotak et al. 2007; Saidi et al. 2011).
These include ROS and the plant hormones ABA, ethylene and salicylic acid. A change in the ROS homeostasis resulting from ROS production and detoxification appears to be a signal. Several mechanisms of ROS sensing have been proposed—for example, unidentified receptor proteins and redox-sensitive transcription factors, including heat shock transcription factors (Mittler et al. 2004). However, neither ROS signalling under heat stress nor the roles of signalling molecules are understood nearly as well as the Hsf-dependent activation of HSP synthesis triggered by protein denaturation.
A second mode of sensing protein denaturation is described for the ER. One of the sensors is an ER membrane-associated transcription factor for chaperones. It is a basic leucine zipper protein, bZIP28 (Srivastava et al. 2012) (Fig. 4.33), with a single-pass transmembrane domain (TMD), a cytosolic N-terminus containing the bZIP element and a C-terminus protruding into the ER lumen. The RRIL site (Arg-Arg-Ile-Leu) on the luminal part serves the processing by specific proteases in the Golgi vesicles. bZIP28 is arrested in the ER by association of its C-terminal domain with the major luminal ER chaperone, the “binding immunoglobulin protein” (BIP).
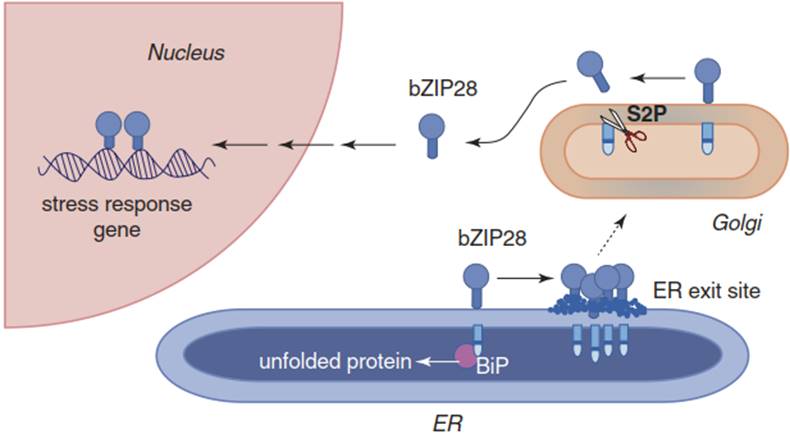
Fig. 4.33. Activation of the bZIP28 transcription factor by stress, such as heat stress. BiP binding protein, a member of the HSP70 family. (Modified from Srivastava et al. (2014))
Upon stress-induced accumulation of misfolded proteins in the ER, BIP is competed away from the C-terminus of bZIP28 and associates with the accumulating misfolded proteins. Dissociation of the bZIP28- BIP complex enables the transcription factor to leave the ER and enter the Golgi. Processing by specific proteases in the Golgi removes the C-terminal part, liberating the cytosolic part of the bZIP28, which can now enter the nucleus as a transcription factor. For binding to the heat shock elements in the promoter of the heat shock genes, oligomerisation of the transcription factor is necessary.
Cell Biological Aspects of the Heat Stress Response. Organisms can acquire thermotolerance by maintaining a high level of HSPs while the normal “housekeeping metabolism” continues. During an acute heat shock, in contrast, the cell redirects its activity to almost exclusive synthesis of HSPs. Several mechanisms contribute to that rearrangement of the cellular processes: the activation of the heat stress transcription factors and the transcription of the unique HSP genes, the inhibition of the processing of the pre-messenger RNAs (pre-mRNAs) of the housekeeping genes combined with preferential translation of the heat stress gene mRNAs, and the conservation of housekeeping pre-mRNA in the nucleus or the surrounding cytosol in the form of heat stress granula (Fig. 4.34).
Since housekeeping mRNAs are not functional under acute heat stress, they would be predestined for rapid degradation by RNAses. By association with small heat stress proteins and HSP70, as well as accumulated HsfA2 protein, these mRNAs are protected from degradation and stored in the multi-chaperone granula of 40 nm diameter. This might promote a return to normal cell physiology after the heat shock or upon development of the acquired thermotolerance. Dissociation of these heat stress granula requires ATP-dependent interaction with further HSPs.
References:
Baniwal SK, Bharti K, Chan KY, Fauth M, Ganguli A, Kotak S, Mishra SK, Nover L, Port M, Scharf K-D, Tripp J, Weber C, Zielinski D, von Koskull-Doring P (2004) Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. J Biosci 29:471-487
Beck E (1994a) Cold tolerance in tropical alpine plants. In: Rundel P, Smith AP, Meinzer FC (eds) Tropical alpine environments: plant form and function. Cambridge University Press, Cambridge. pp 77-110
Beck E (1994b) Turnover and conservation of nutrients in the pachycaul Senecio keniodendron. In: Rundel P, Smith AP, Meinzer FC (eds) Tropical alpine environments: plant form and function. Cambridge University Press, Cambridge. pp 215-222.
Beck E (2011) Abiotischer Stress pur: Uberlebensstrategien von (tropischen) Hochgebirgspflanzen. Rundgespr. Kommission fur Okologie, Bayer. Akad Wissensch 39:93-110
Beck E, Heim R, Hansen J (2004) Plant resistance to cold stress: mechanisms and environmental signals triggering frost hardening and dehardening. J Biosci 29: 449-459
Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4:273
Bravo LA, Griffith M (2005) Characterization of antifreeze activity in Antarctic plants. J Exp Bot 56:1189-1196
Brush RA, Griffith M, Mlynarz A (1994) Characterization and quantification of intrinsic nucleators in winter rye leaves. Physiol Plant 104:725-735
Buchanan BB, Gruissem W, Jones RL (2015) Biochemistry and molecular biology of plants, 2nd edn. Wiley, Hoboken
Date added: 2025-01-18; views: 372;
