Sensing of Flooding and Ensuing Signal Transduction
Elongation growth, programmed cell death and metabolic adjustments all represent acclimations and modifications that are activated upon waterlogging and/or submergence, and often depend on changes in gene expression. This clearly implies the existence of sensing and signalling mechanisms. The gaseous hormone ethylene plays a central role as a response mediator under O2 deficiency. The key modifications—aerenchyma formation, adventitious root emergence, hypo- nastic growth and stem elongation—are all controlled by ethylene as the trigger.
Ethylene is constitutively produced in all cells of a plant. Upon flooding, ethylene immediately (within 1 h) accumulates in and around roots and submerged shoots because of the strongly reduced gas exchange under water. Thus, it represents an early and very reliable indicator of flooding. As detailed in Sect. 5.6, ethylene is employed for controlling contrasting strategies via the regulation of differential gene expression through ethylene response factors (ERFs).
Ethylene Signal Transduction. The simple alkene ethylene regulates a multitude of developmental processes in plants, including seed germination, leaf abscission and fruit ripening, as well as many responses to abiotic and biotic stresses. The ethylene signalling pathway has been elucidated in Arabidopsis thaliana (Fig. 5.12).
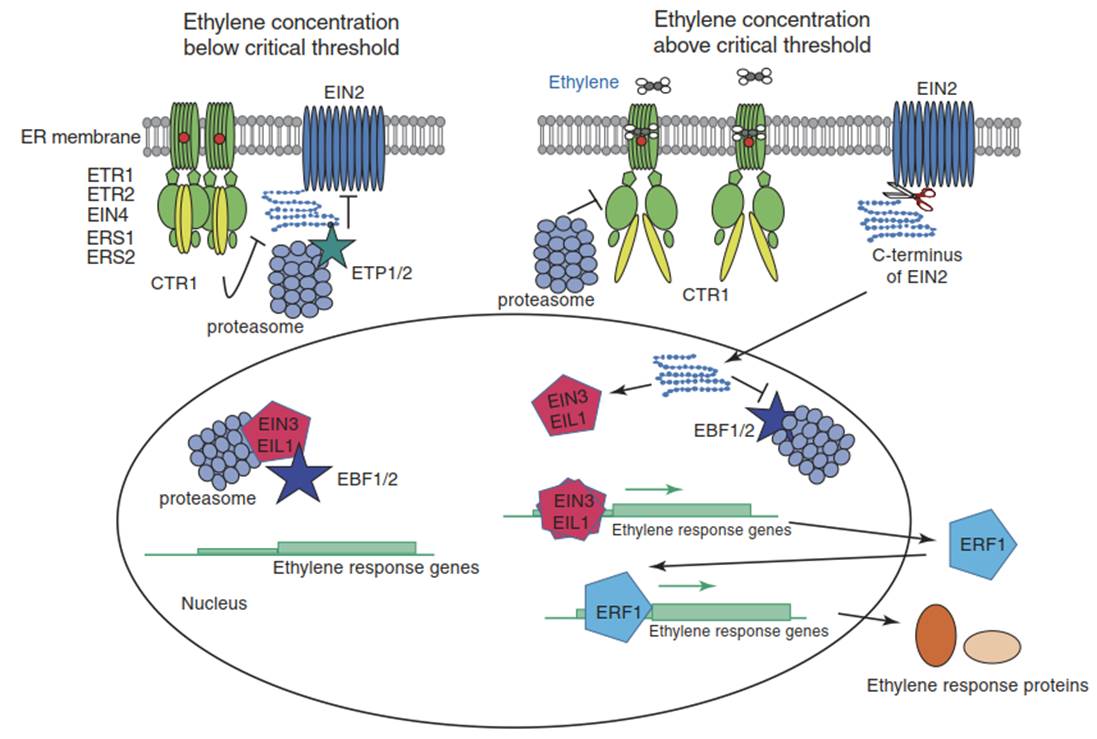
Fig. 5.12 Ethylene signal transduction. According to the current model of ethylene signal transduction in Arabidopsis thaliana, the five ethylene receptors (ETR1, ERS1, ETR2, ERS2 and EIN4) (shown as green structures; two receptors are shown exemplarily) reside in the membrane of the endoplasmic reticulum as homodimers. Copper (shown as red circles) serves as a cofactor for ethylene binding. The ethylene receptors are negative regulators. In the absence of a critical ethylene concentration (left side), the receptors activate the kinase CTR1 (shown in yellow), which suppresses the response.
The positive regulator EIN2 (shown in blue) is inactivated by phosphorylation through CTR1 and is tagged for degradation in the 26S proteasome by the F-box proteins ETP1 and ETP2. Two other F-box proteins, EBF1 and EBF2, mediate degradation of the transcription factors EIN3 and EIL1 (shown in red) in the nucleus. No transcription of the ethylene response genes occurs.
When the ethylene concentration rises above a critical threshold—for example, because of strongly reduced diffusion out of plant tissues into flooded soil—the receptors bind the hormone and become inactivated. This switches off CTR1 and prevents the phosphorylation of EIN2. The C-terminal end of EIN2 moves to the nucleus after cleavage, stabilises the transcription factors EIN3/EIL1 and induces degradation of EBF1/2.
The transcription factors dimerise and bind to cis elements in the promoters of ethylene response genes such as ERF1, thereby activating their expression. ERF1 and other products of early genes then activate expression of hundreds of additional ethylene response genes. Their combined activities bring about acclimative changes in morphology and metabolism. (Modified from Merchante et al. (2013))
In dark-grown A. thaliana seedlings, ethylene induces the so-called triple response: an increased apical hook of the cotyledons, thickening of the hypocotyl instead of extension growth, and reduced root elongation. The triple response is easy to score and allows the isolation of mutants showing either ethylene insensitivity (etr, ein) or constitutive responses in the absence of ethylene (ctr). Molecular analysis of these mutants has defined the core pathway of ethylene signalling, which has since been found to be highly conserved in the plant kingdom.
The ethylene signal is perceived by ethylene receptors. They share sequence similarity with the bacterial two-component histidine kinases, which indicates their evolutionary origin. Interaction with the extremely simple ligand C2H4 requires Cu as a cofactor. Apparently, all terrestrial plants, including mosses, possess several receptors that can homodimerise and form higher order complexes. A. thaliana has five ethylene receptors, with ETR1 being the most studied. Ethylen receptors are negative regulators that suppress responses in the absence of the signal. They do this by activating another negative regulator, the serine/threonine kinase CTR1, which inactivates the next downstream component, EIN2, through phosphorylation of its C-terminus (Fig. 5.12).
Ethylene receptors reside in the membrane of the endoplasmic reticulum. This is possible because ethylene freely diffuses through aqueous and lipid phases. Upon binding of the ligand, the receptors become inactivated and switch off CTR1. The positive regulator EIN2 is thus no longer phosphorylated, which triggers cleavage of the C-terminus of EIN2, its movement into the nucleus and the triggering of the transcriptional cascade constituting the ethylene response. The EIN2 C-terminus stabilises the transcription factors EIN3 and EIL1, which in the absence of ethylene are tagged for proteasomal degradation by EBF1 and EBF2. EIN3/EIL1 dimerise and activate transcription of genes encoding transcription factors such as the ERFs (e.g. SUB1A in rice; Fig. 5.14 and Box 5.1), which then activate hundreds of other ethylene-responsive genes.
This linear core pathway is modulated by various additional mechanisms. For instance, Cu supply to the ethylene receptors is dependent on the Cu-ATPase RAN1. More recently discovered regulatory components promote, for example, the transition of the ethylene receptors from the inactive to the active state (RTE1), or they influence the stability of EIN2 (ETPs) (Merchante et al. 2013).
Oxygen Sensing. A second indicator of flooding is, of course, the O2 status. However, while ethylene concentrations rapidly increase in all organs upon submergence, the situation is more complex for O2. In contrast to root cells, which can become anoxic quite rapidly, shoot O2 levels can show a pronounced diurnal pattern with comparatively high concentrations during the light period because of photosynthetic O2 generation (Voesenek and Sasidharan 2013). The existence of a direct oxygen-sensing mechanism in plants has long been debated. The alternative scenario postulated an indirect sensing of O2 levels through the perception of, for instance, the energy charge of cells or the cytosolic pH. However, an oxygen sensor was finally discovered in A. thaliana (Licausi et al. 2011; Gibbs et al. 2011).
It regulates ethylene response factors, which are important for low-oxygen survival. In this way the two important indicators of flooding stress are integrated. It is important to note, however, that acclimations and modifications differ with respect to the relative roles that ethylene and low O2 play in activating them. Overall, oxygen sensing is mainly important for metabolic changes, while ethylene signalling has a broader role and is essential for most responses upon flooding, including morphological modifications.
The sensor uses the oxygen dependence of the amino (N)-end rule pathway for targeted proteolysis of proteins that carry a cysteine at the N-terminus right after the first amino acid, methionine (Fig. 5.13). Following cleavage of the methionine, the cysteine can be oxidised enzymatically. This enables arginylation, that is, the addition of an arginine residue, which tags the proteins for proteasomal degradation.
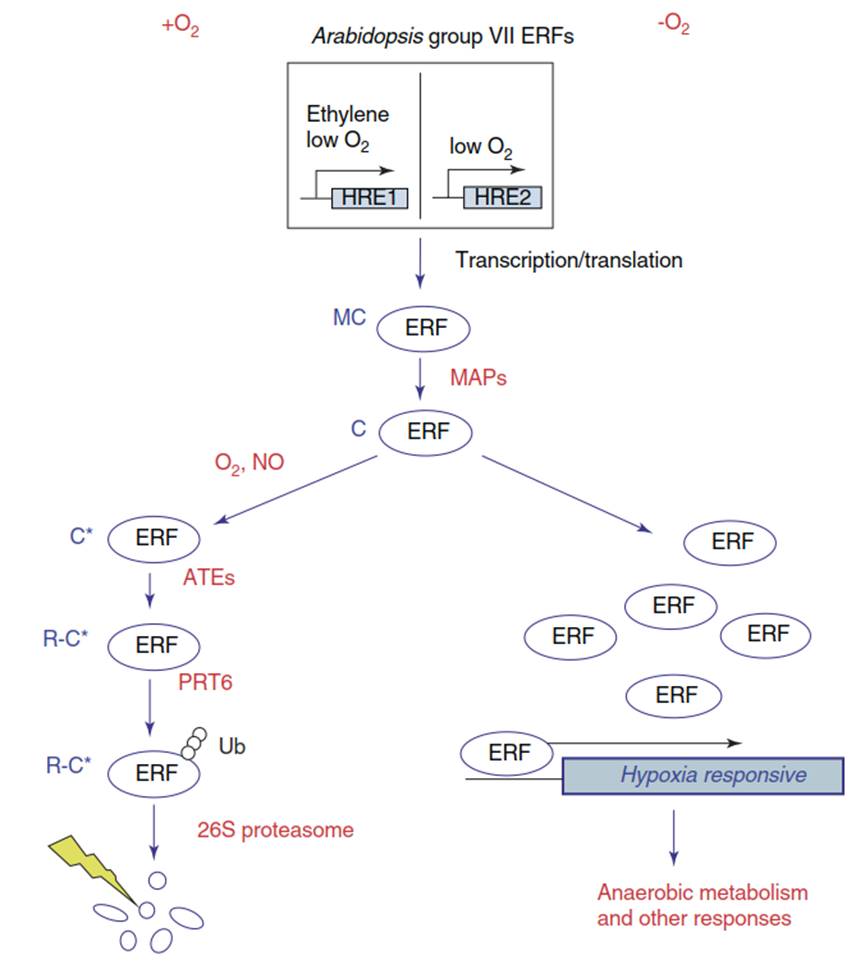
Fig. 5.13. The oxygen sensor in Arabidopsis thaliana. Under hypoxic conditions, ethylene signalling activates transcription of group VII ethylene response factors (ERFs; Fig. 5.12). These ERFs (HRE1 and HRE2 are shown as examples) all share the N-terminal sequence Met-Cys (MC) and are thus substrates of the N-end rule pathway. Methionine (M) is cleaved by a methionine aminopeptidase (MAP). In the presence of O2 (normoxic conditions) or NO, the cysteine is oxidised. After addition of an arginine (R) by arginyl transfer RNA (tRNA) transferase (ATE), the protein is recognised by the E3 ligase PRT6, which tags the arginylated ERF for degradation in the 26S proteasome by adding several ubiquitins (Ub). Thus, the ERFs cannot activate expression of ethylene response genes. However, when the cellular O2 concentration drops, the cysteine oxidation eventually cannot occur. ERFs are not degraded and now activate the hypoxia responses. (Modified from Bailey-Serres et al. (2012a))
Thus, under normoxic conditions in a cell, these proteins are destabilised. Among the proteins with a methionine-cysteine combination at the N-terminus in A. thaliana are the class VII ERFs, which mediate various hypoxia responses. Thus, they are rapidly degraded when O2 for the cysteine oxidation is available and cannot activate hypoxia responses. In contrast, under low-oxygen conditions, this oxidation of the cysteine no longer occurs, resulting in greater stability of the response factors and the activation of metabolic and developmental changes supporting survival of flooding or submergence.
Date added: 2025-01-18; views: 350;
