Water Deficiency (Drought). The Properties of Water
Water accounts for up to 90% of a plant’s fresh weight. Furthermore, since the experiments of Stephan Hales in the eighteenth century it has been known that a large fraction of the water taken up by plants from the soil is lost to the air, that is, it is transpired. This water loss is an inevitable consequence of the need for terrestrial plants to take up CO2 from the atmosphere. For every molecule of CO2 that enters a plant through stomata by diffusion, several hundred molecules of H2O leave the plant via this same pathway.
The concentration gradient across the leaf surface is much greater for water loss than for CO2 uptake, but a membrane or other material that would allow selective passage of CO2 has never evolved in plants. Thus, not only is water the most abundant of the resources needed by a plant for functioning and growth—a characteristic that plants share with animals—but CO2 uptake and thereby photosynthesis require large fluxes of water through the plant, which is the reason why water availability very often limits productivity. The strict correlation between CO2 uptake and water loss is sometimes referred to as the central dilemma of plants: dying of thirst or dying of hunger?
A second distinctive feature of plant-water relations is based on a major difference in the structure of plant and animal cells. Plant cell walls can withstand considerable hydrostatic pressures and tensions. The ability to build up turgor pressure is essential for growth via cell expansion and for the rigidity of tissues not stabilised by lignified cells.
Besides temperature, precipitation is the most dominant environmental factor determining the distribution of vegetation on the global scale. Vast differences exist between plant species in the ability to grow and reproduce in water-limited habitats, estimated to represent more than 50% of the Earth’s surface area. Following a consideration of the unique properties of water, this chapter will address cellular aspects of plant-water relations such as the driving forces for water movement, the water conductivity of membranes and cellular responses to water scarcity. Plants synthesise a range of protective molecules, regulate their osmotic potentials and—most importantly—control the rate of water loss through stomata (Fig. 6.1).
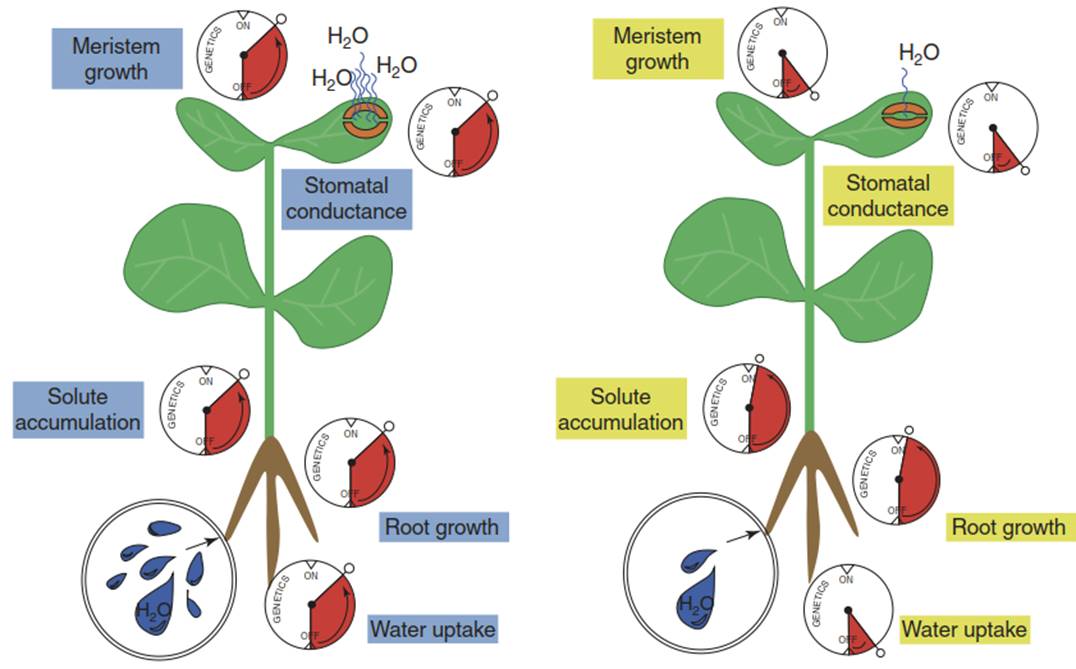
Fig. 6.1. A plant before (left) and during (right) drought stress. Major control points of acclimation are indicated as valves. (Modified from Maggio et al. (2006))
Also covered are the molecular mechanisms underlying growth responses to drought and the photosynthesis variants (C4 photosynthesis and Crassulacean Acid Metabolism (CAM)) that result in higher water use efficiency, i.e., a more favourable ratio of water loss to CO2 fixation. Plant-water relations at the whole-plant level are described in Chap. 10.
The Properties of Water. The biochemistry of life requires water in the liquid state and is thus dependent on the physicochemical properties of the water molecule. These are often referred to as anomalies (Box 6.1) and result from the dipole nature of the molecule Hδ+-Oδ--Hδ+ (Fig. 6.2a). The water molecule is bent (at an angle of around 104°) and therefore has an asymmetrical charge distribution. The dipoles produce hydrogen bonds between the individual molecules, and this guarantees a high degree of cohesion with, at the same time, low viscosity.
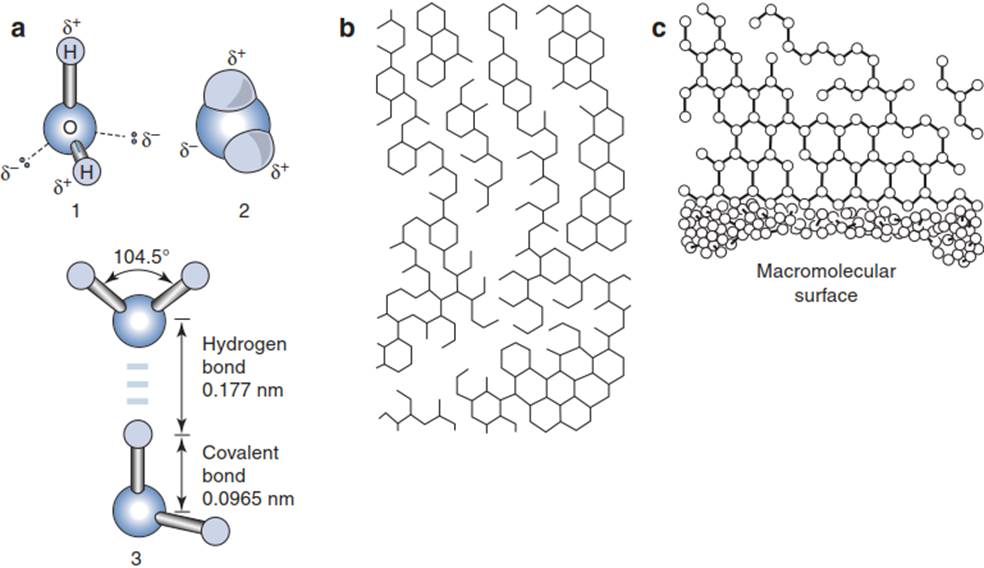
Fig. 6.2. Dipole, clusters, hydrophobic interaction
The water molecules form flickering (mobile) clusters or aggregates, which continuously exchange individual molecules. That explains why water is in the liquid rather than the gaseous state at temperatures between 0 and 100 °C and at standard air pressure, despite the low molecular mass of the water molecule. Related consequences of the dipole nature of water are adhesion to polar surfaces such as cell walls, and capillary forces caused by the high surface tension. Adhesion, capillary forces and cohesion, as well as low viscosity, are decisive factors for the transport of water from roots to leaves (the cohesion-tension theory of water conductance; Chap. 10 and plant physiology textbooks).
Another consequence of the dipole nature of the water molecule is its suitability as a solvent for polar and polarisable compounds. In addition, water exerts a structuring force in amphiphilic systems (hydrophilic-hydrophobic) giving rise to lipid micelles and contributing to the tertiary structures of proteins. Furthermore, water is a very effective heat buffer for organisms because of its relatively high heat of crystallisation (freezing avoidance) and very high heat of vaporisation (transpiration cooling). As its radiation absorption is outside the boundaries of the visible spectrum, water does not absorb visible light and thus does not interfere with photosynthesis or processes regulated by blue or red light.
Date added: 2025-01-18; views: 332;
