Water Acquisition and Movement: Cellular Aspects
Life is so strictly dependent on water that the search for traces of extraterrestrial life (e.g. on Mars) is essentially a search for signs of water. The evolution of life on land has required key innovations allowing organisms to acquire water. In addition, most animal and plant species have to maintain a hydrated state; that is, they are homoiohydric. Only few species of vascular plants can withstand dehydration and are able to resume physiological activity after rehydration. Together with lichens and many mosses, they are referred to as poikilohydric.
The needs to take up water and to stay hydrated demand acclimations and adaptations to cope with fluctuations in water supply and, in particular, with a shortage of water supply. Many plant habitats are characterised by either temporary or permanent water scarcity. Not only does this threaten photosynthesis and the functions of all kinds of metabolic processes that take place in the aqueous environments of cells, but water is also physically important for the growth of plants. Growth is a function of cell division and cell expansion. The expansion growth of plant cells is dependent on turgor pressure, i.e. on water influx. The modulation of cell wall properties such as their extensibility controls the expansion rate, but the build-up of hydrostatic pressure is what drives the expansion.
Thus, practically every facet of terrestrial plant life is dependent on the uptake of water from the soil at sufficient rates and the tightly controlled movement of water within the plant. Water uptake by roots and water movement are dependent on a driving force—that is, pressure differences—and on the facilitation of passage through biological membranes. We consider cellular water homeostasis and short-distance transport here. Long-distance transport of water is covered in Chap. 10.
The Physico-chemical Properties (Anomalies) of Water. Because of their dipole nature, water molecules (Fig. 6.2a) associate via hydrogen bonds to a three-dimensional lattice (clusters, Fig. 6.2b), which is in a permanent molecular rearrangement. The physico-chemical anomalies of water (e.g. high melting and boiling temperatures in comparison with molecules such as H2S or ethanol, Table 6.1) can be attributed to this cluster formation.
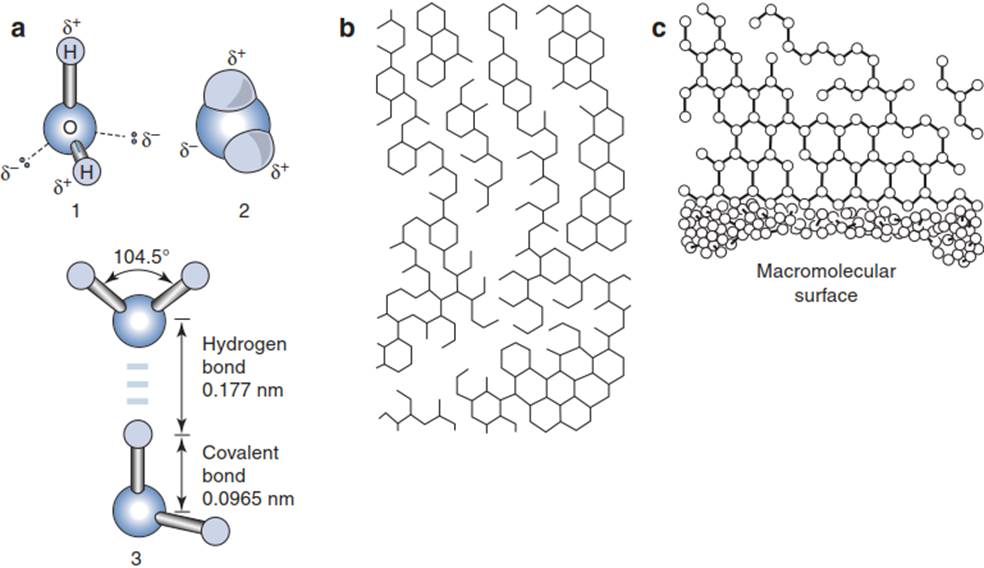
Fig. 6.2. Dipole, clusters, hydrophobic interaction

Table 6.1. Physico-chemical anomalies of water
The properties of water are also the reason for hydrophobic interactions. Non-polar molecules in an aqueous environment are forced into aggregates in order to minimise the energetically unfavourable interaction with water molecules. Amphiphilic molecules (i.e. molecules with polar and non-polar groups) such as phospholipids and glycolipids form ordered structures (= biomembranes) with the hydrophilic part interacting with water molecules and the hydrophobic part excluding water (Fig. 6.2c) (Larcher 2003).
Date added: 2025-01-18; views: 355;
