Regulation of the Stomatal Aperture
Leaf surfaces sealed by a cuticle and wax deposition represent a key innovation for the evolution of land plants. Without an effective barrier against water loss the maintenance of a relatively constant internal water status (homoiohydry) would not be possible in most terrestrial habitats. While there is considerable variation in the effectiveness of the sealing—for example, between plants inhabiting wetlands and xerophytes thriving in arid habitats—the cuticular water conductance rarely accounts for more than a small fraction (10% or less—much less in the case of xerophytes with a thick cuticle and massive wax deposition) of total evaporation.
Most of the gas exchange and, with that, most of the water loss are due to stomatal conductance. It has been estimated that about 60% of all terrestrial rainfall globally is returned to the atmosphere through stomata. In water-limited ecosystems this proportion can be even higher. Because of the large difference in water potential between leaves and air (with the air having very negative values), the control of the stomatal aperture is the most important response to conditions of low water availability—for example, a more negative soil water potential. Regulation of stomatal conductance therefore plays a key role in a plant’s response to water deficit and drought stress tolerance. Plants that are unable to close their stomata die quickly when water is withheld.
Several internal and environmental cues are integrated by guard cells (which form the stomatal pore) in order to optimally adjust the stomatal aperture for any given physiological situation. Stomatal opening is in most plants (with the exception of CAM plants; Sect. 6.6) triggered by light. Stomatal closure under conditions of water limitation is elicited by the phytohormone abscisic acid (ABA) (Fig. 6.12). Other factors influencing the stomatal aperture are temperature (with lower temperatures favouring opening) and internal CO2 (with higher CO2 partial pressure favouring closing). Recognition of microorganisms via microbe-associated molecular patterns leads to stomatal closure because stomata represent important entry sites for pathogens.
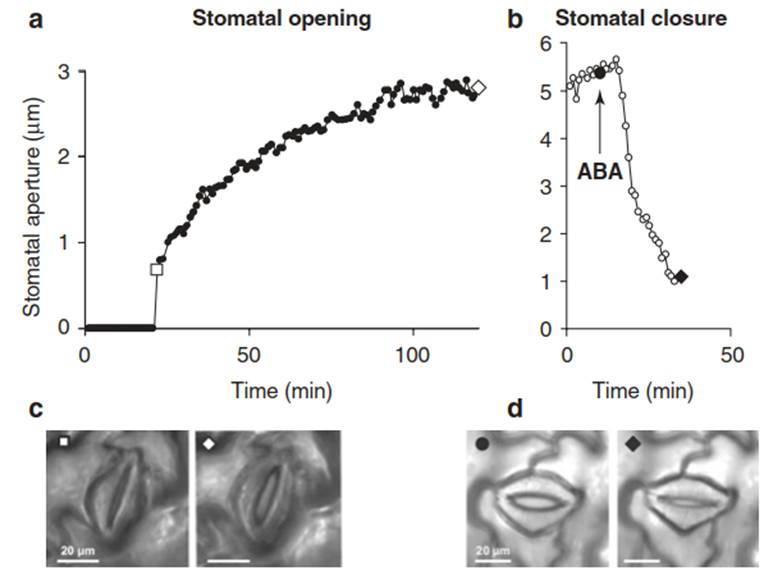
Fig. 6.12. Light-induced and abscisic acid (ABA)–induced stomatal movement in the abaxial epidermis of intact tobacco leaves. a At t = 0 the leaves were illuminated with white light. The images shown in c correspond to the open symbols on the graph in a. b ABA (10 μM) was applied to the leaf cuticle. The images shown in d correspond to the closed symbols in b. Note the different response kinetics. (Kollist et al. 2014)
The stomatal aperture is nearly linearly correlated with the guard cell turgor pressure. Guard cells are built in such a way that higher turgor pressure leads to a bending of the cells and thereby an opening of the pore between them. The turgor pressure is a function of the osmotic potential of the cells. A more negative value relative to the apoplast results in water influx, and vice versa. Thus, the concentration of solutes in guard cells determines stomatal conductance. Stomatal movement is driven by the transport, as well as the synthesis and degradation, of solutes. Most important are K+ ions and their counter ions Cl- and malate2-.
Changes in K+ and Cl- concentrations are brought about by ion channel-mediated exchange between guard cells and the surrounding apoplast, as well as between guard cell vacuoles and the cytosol. Malate, in contrast, is either synthesised from starch or degraded via mitochondrial respiration either in guard cells or in neighbouring epidermal cells. Because the majority of solutes are stored in the vacuoles, the control of stomatal movement depends on the modulation of transport activities in both the plasma membrane and the tonoplast.
The principal classes of transporters are described in Chap. 7. Negative membrane potentials across the plasma membrane and the tonoplast are generated by different types of proton pumps: P-type H+-ATPases in the plasma membrane and V-type ATPases and pyrophosphatases in the tonoplast (Fig. 6.13).
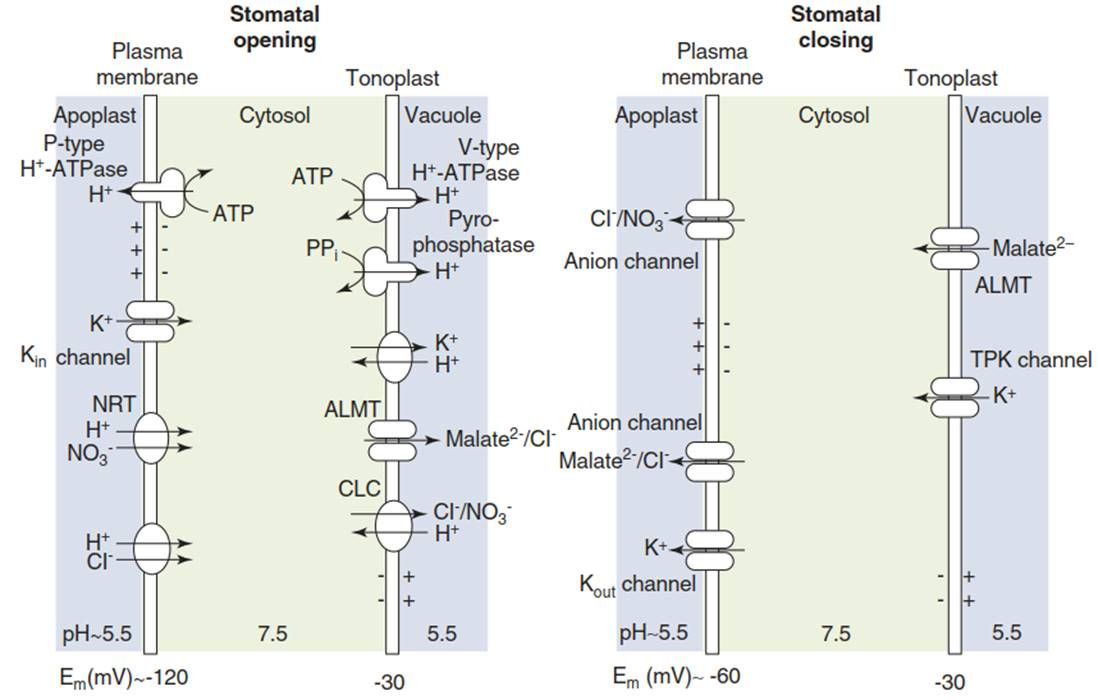
Fig. 6.13. Plasma membrane-localised and tonoplast-localised channels and transporters involved in opening and closing of stomata. Proton pumping activities of P-type H+-ATPases, V-type H+-ATPases and pyrophosphatases result in an acidic pH of the vacuole and the apoplast. Opening of stomata is triggered by a stimulation of proton pumping that renders the potential of the plasma membrane more negative (approximately -120 mV), which activates inward-rectifying K+ channels (Kin channels). Influx of anions is dependent on H+- driven symporters such as the NRTs in the case of NO3-.
The K+ ions taken up into the cytosol are transported into the vacuole by NHX transporters—antiporters driven by H+ flux. Anions and malate are stored in the vacuole, following passage through anion channels (ALMT) or transporters (CLC). Stomatal closure is initiated by the activation of anion channels. The efflux of anions out of the cell depolarises the plasma membrane to about -60 mV. Outward-rectifying K+ channels open and K+ efflux occurs. (Modified from Kollist et al. (2014))
K+ influx and efflux is mediated by K+ channels, whose activity is dependent on the plasma membrane potential. Inward-rectifying channels (i.e. channels allowing the passage of ions more easily into the cell) open at membrane potentials more negative than the resting potential for K+ (i.e. upon hyperpolarisation) and mediate K+ influx. Outward-rectifying K+ channels (i.e. channels allowing the passage of ions more easily out of the cell) open at membrane potentials more positive than the resting potential for K+ (i.e. upon depolarisation) and mediate K+ efflux. K+ uptake into the vacuole occurs against a concentration gradient and the electrical potential difference. Thus, it is assumed to require K+/H+ symport. Efflux from the vacuole is channel mediated.
Because of the voltage dependence of K+ channels in the plasma membrane, K+ movement into and out of guard cells is controlled by the plasma membrane potential. Hyperpolarisation is achieved by increases in proton pumping activity. Light-triggered stomatal opening is dependent on blue light receptors (phototropins), which further activate H+-ATPases, leading to hyperpolarisation, K+ influx and, finally, osmotically driven water uptake.
There is also evidence for an opening in response to photosynthetically active radiation, which is mechanistically poorly understood. The actual signal sensed by the guard cells could be the lowering of the CO2 concentration in the sub-stomatal cavity owing to active photosynthesis. In this way, CO2 demand would be coupled to the stomatal aperture.
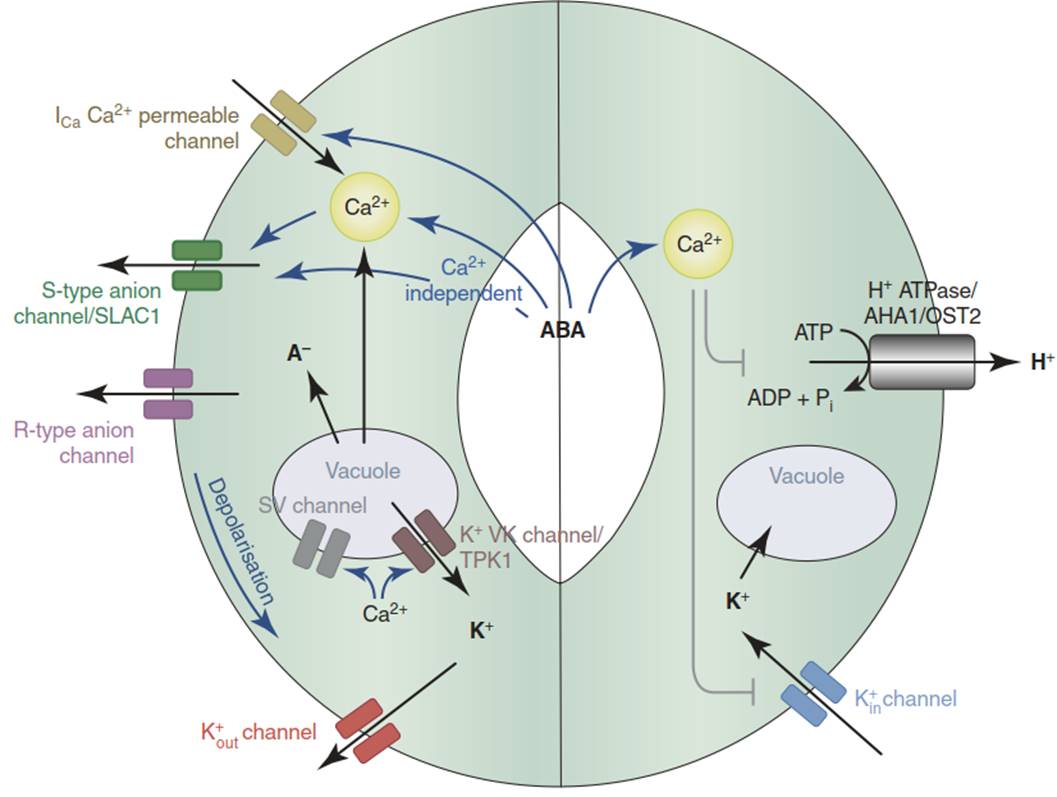
Fig. 6.14. Abscisic acid (ABA)–dependent guard cell ion channel regulation. ABA triggers stomatal closure (shown on the left) and inhibits stomatal opening mechanisms (shown on the right), which are activated, for instance, by blue light. AHA1 ARABIDOPSIS H+ ATPASE 1, ICa inward Ca2+ current, OST2 OPEN STOMATA 2, R-type rapid-type, SLAC1 SLOW ANION CHANNEL 1, S-type slow-type, SV slow vacuolar, TPK1 TWO PORE K+ CHANNEL 1, VK vacuolar K+ selective. (Kim et al. 2010)
ABA-triggered stomatal closure is mediated by an inactivation of proton pumps and a concomitant activation of Cl- channels (such as SLAC1) in the plasma membrane. The resulting depolarisation opens the outward-rectifying K+ channels and thereby causes K+ efflux and water loss into the apoplast (Fig. 6.14). ABA signal transduction is discussed in Sect. 6.5. Other factors triggering stomatal closure include low humidity and elevated atmospheric CO2 levels.
Date added: 2025-01-18; views: 348;
