Protective Proteins
The function of proteins accumulating under conditions of drought have been mostly studied in the context of desiccation—that is, a lowering of the relative water content of a tissue or organ to 10% or less. Desiccation tolerance represents an adaptation to extreme environmental conditions and requires specific mechanisms displayed by only a small number of plant species (around 300 within the angiosperms).
This makes it different from the acclimative control of osmotic potential or the stomatal aperture, which essentially every plant is capable of. It involves a state of dormancy of the whole plant, which is reminiscent of seeds. In fact, it has been proposed that in resurrection plants the developmental programmes underlying seed maturation have been recruited for the desiccation tolerance of the whole plant (Farrant and Moore 2011).
During the early phase of dehydration, osmotic adjustment occurs through the accumulation of sucrose and other osmolytes. Progressive water loss concentrates the cellular content and causes mechanical as well as metabolic stress. The risk of protein denaturation and unwanted biochemical reactions increases. Osmolytes replace water molecules and can eventually accumulate to levels such that vitrification occurs; that is, the sucrose solution adopts a glassy, almost solid state (Fig. 6.10). Proteins that have the capacity to protect cellular structures strongly accumulate.
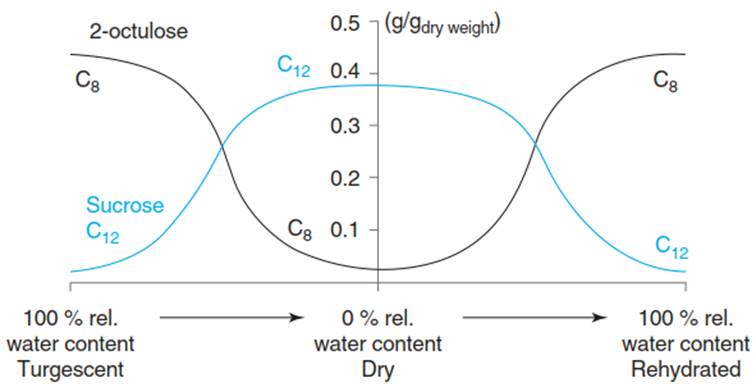
Fig. 6.10. Changes in the proportions of soluble carbohydrates, corresponding to the water relations of the resurrection plant Craterostigma plantagineum. In the fully hydrated state, leaves mainly contain the C8 sugar octulose which, upon desiccation, is almost quantitatively converted to sucrose, a compatible solute. This reaction is reversible. (After Bartels et al. (1993))
They are called late embryogenesis abundant (LEA) proteins. The name indicates that these proteins were first described as accumulating during the later stages of seed maturation, when desiccation tolerance is acquired. However, they are found not only in plants but also in bacteria, archaea, fungi and certain invertebrates (e.g. nematodes, arthropods).
Within the plant kingdom, LEA proteins are not specific to resurrection plants. They accumulate in the seeds of most other plants during ripening. Also, their expression is induced under conditions of water limitation such as drought, freezing and high salt concentrations in the vegetative tissue of many non-desiccation-tolerant plant species. Some of the COR (COld-Regulated) genes up-regulated during cold acclimation encode LEA proteins.
Genome-wide analyses in model species have shown that different subsets of LEA genes are expressed during seed maturation and abiotic stress with very little overlap. Resurrection plants (Fig. 6.11) are special with respect to LEA proteins only in that they often show much stronger accumulation of these proteins. Also, in some species, these proteins are constitutively expressed at high levels even in the absence of water deficit. The latter is interpreted as a way of priming these plants for dehydration upon the arrival of severe drought events.

Fig. 6.11. The resurrection plant Craterostigma plantagineum, which recovers fully from drying out: a plant in the hydrated state (left), in the dry state (centre) and after rehydration (right). (Photos courtesy of Dorothea Bartels, University of Bonn, Germany)
LEA proteins are highly hydrophilic. On the basis of their amino acid composition (for instance, a high content of glycine and other small amino acids) and supported by biochemical investigations of a few examples, most of them are assumed to be intrinsically disordered proteins—that is, lacking a tertiary structure. Large numbers of genes encoding LEA proteins are found in plant genomes (>50 in A. thaliana, for example). LEA proteins are divided into several classes according to sequence similarities. The annotation and categorisation of LEA proteins are not consistent throughout the literature. They largely overlap with another group of proteins named “hydrophilins”, which are defined as glycine rich and hydrophilic. Among the different classes of LEA proteins there are some that carry alternative names such as “dehydrins”.
The increase in LEA protein abundance under water deficit conditions has been documented for a large number of plant species, organs, tissues and cell types. LEA proteins have thus been firmly associated with abiotic stress tolerance. Still, the functional understanding of LEA proteins is limited. How exactly their accumulation promotes cell survival under stress is unknown (Wise and Tunnacliffe 2004). Enzymatic activities have never been described. Many LEA proteins become structured—that is, partially folded—upon dehydration and may exert their protective effects in this state.
On the other hand, the accumulation in water-limited vegetative tissues not undergoing desiccation suggests functions of LEA proteins also in the hydrated state. Several hypotheses exist as to the actual physiological and biochemical activities, including the stabilisation of proteins and membranes, anti-oxidative activities or a function as space-filling molecules in cells with low water content to prevent collapse.
In vitro it has been shown for several enzymes that the presence of LEA proteins preserves activity, probably because the hydrophilicity of LEA proteins prevents the formation of protein aggregates. This is sometimes referred to as a “molecular shield” mechanism and is different from the chaperone activity of heat shock proteins, as LEA proteins cannot protect proteins from heat denaturation. According to this hypothesis the unstructured LEA proteins would sterically hinder the interaction between partially denatured proteins.
The anti-aggregate function is supported by in vivo data obtained for cells coexpressing LEA proteins and aggregation-prone target proteins. Evidence for an interaction of LEA proteins with membranes exists as well. Binding may occur concomitantly with the formation of a-helices upon drying or alternatively in a hydrated state. Certain dehydrins have been found to electrostatically interact with membrane lipid head groups in solution. Direct evidence for stress protection conferred by LEA proteins is rare. Numerous studies with plants ectopically expressing LEA proteins have reported comparatively modest gains in stress tolerance.
Besides LEA proteins, other protective proteins accumulate in resurrection plants and generally in plant cells affected by water loss. The most prominent ones are small heat shock proteins (sHSPs). They show true chaperone characteristics in addition to a general stabilising effect on cellular macromolecules and membranes. A third group of protective proteins comprises ROS scavenging enzymes such as aldehyde dehydrogenases and peroxiredoxins to counteract the production of ROS in cells affected by water loss.
Date added: 2025-01-18; views: 303;
