Nanomaterials in Water: Detection and Characterization. Analytical Methods
The presence of nanomaterials in water can be determined either indirectly, by identifying changes in bulk properties introduced by nanomaterials, or directly, by identifying individual nanomaterials. Indirect methods of detection involve the measurement of bulk properties, such as elemental concentrations, elemental ratios, isotopic ratios, crystal structure, and element complexation. Depending on the type of nanomaterial and the aquatic system under investigation, changes in these properties can be reasonably attributed to the presence of nanomaterials. Direct methods of detection involve measurements of particle number, size, shape, and composition. Such measurements provide direct observations of nanomaterials, but are hindered by the complexity of sample handling. In order to prevent transformations such as agglomeration, de-agglomeration, dissolution, or interactions with other components of the sample, each analytical technique requires appropriate sample preparation strategies.
Bulk Analysis. Bulk analysis is a two-step process: first, solids are separated from the water by means of filtration, centrifugation, cloud-point extraction, centrifugal ultra-filtration, etc. The separation method depends on the characteristics of the nanomaterial under investigation and the characteristics of the water sample. Second, the solids are analyzed by techniques, such as inductively coupled plasma mass spectrometry (ICPMS) or inductively coupled plasma optical emission spectrometry (ICPOES), X-ray absorption spectroscopy (XAS), X-ray diffraction (XRD), X-ray fluorescence (XRF), and others. XRF, ICPMS and ICPOES are used to quantify the concentration of elements, XRD is used for identifying mineral structures, and XAS can be used to measure the complexation and coordination chemistry of elements. The sensitivity of these techniques to the presence of nanomaterials depends on the ratio of nanomaterial mass to the total solids mass of the sample and the instrumentation uncertainty (Table 1).
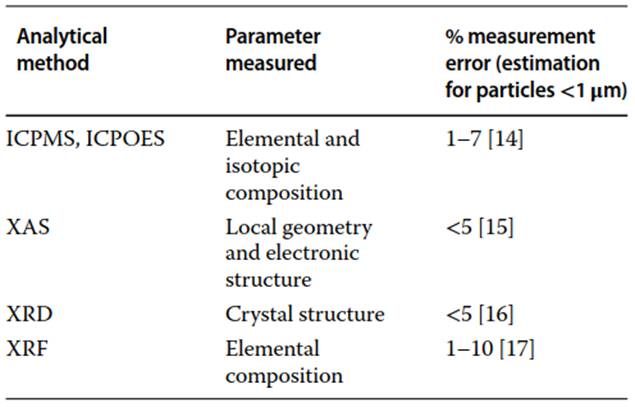
Table 1. List of analytical methods for bulk analysis
In most cases, a significant amount of nanomaterials is necessary to overcome the analytical uncertainty and therefore, this approach can only be applied in scenarios of intense contamination with MNMs, i.e. typically more than 1% by weight of the total solids in the sample. MNMs produced in large volumes are more likely to be involved in such cases, e.g. the release of silicon dioxide or titanium dioxide nanoparticles from sunscreens, textiles, or paints. Information about elemental sample composition (qualitative and quantitative) adds value to more detailed and targeted analysis of nanomaterials.
Microscopy. Microscopy is a technique favored in several scientific disciplines, because it provides visual representation of the sample, often combined with additional information, such as fluorescence, elemental composition, etc. For nanomaterial analysis, particles are typically separated from the water media, by filtration or centrifugation. A small volume (10-50 µL) is then deposited or centrifuged on a flat surface and dried. Sample treatment is not the focus of this article; however, it is noted that the sample preparation is dictated by the sample characteristics and the conditions that will be used during analysis, mainly high vacuum, variable vapor pressure, or cryogenic electron microscopy. The small size of nanoparticles requires that microscopes with high resolution are used, so that the morphological limits of each particle can be defined with adequate accuracy. The most commonly used instruments are electron and atomic force microscopes.
Date added: 2025-02-13; views: 295;
