Discussion. Challenges with Environmental Samples
The analysis of MNMs in environmental samples is not a trivial task because MNMs are likely to be released gradually, in low numbers, and diluted into large and mobile water bodies, such as creeks, rivers, lakes, and oceans. While MNMs are diluted in these waters, they are also mingled within a multitude of natural particles of various sizes and compositions that together with natural organic substances comprise the SPM in natural waters. In such environments, MNMs may aggregate with SPM, attach on solid surfaces, or undergo surface reactions, such as dissolution, adsorption, and chemical reactions that will alter the physicochemical characteristics of the MNMs.
Therefore, the sample collection and analysis strategy need to take into account these possibilities. Analytical techniques, such as light scattering, TEM, atomic force microscopy, etc. that are routinely used to characterize MNM suspensions following their synthesis or in laboratory test media are not capable of dealing with the low MNM concentrations and complexity of environmental samples as standalone methods. However, these techniques may be used in combination with sample treatment methods, such as pre-concentration and fractionation. An overview of the aforementioned nanometrology techniques is given in Table 2.

Table 2. List of analytical methods for bulk analysis
Techniques are ranked with a scale of low, medium, and high, according to their ability to measure number concentration, particle composition, size, shape, and their elemental selectivity. Based on the experience of the authors, a rough estimation is made for the potential of each method to be applied for environmental nanometrology purposes.
Examples and General Strategy. As the electron density of an element increases, so does the ease of detection. For example, silver nanoparticles will have a lower size detection limit with spICPMS than titanium dioxide nanoparticles [68]. Lighter elements are both harder to detect and are more abundant in nature. Thus, their analysis is likely to be time and resource consuming and necessitate the acquisition of multiple information. A typical example is the detection of titanium dioxide nanoparticles in lake water. Titanium is an abundant element in nature and forms weathering resistant oxides, such as anatase, brookite, and rutile (TiO2), which may occur in various sizes, including nanoparticles. It is also a component in several minerals, such as ilmenite (FeTiO3), perovskite (CaTiO3), and sphene (CaTiSiO5).
The detection of anthropogenic TiO2 nanoparticles is therefore complicated by the various sources and seasonal variations of natural minerals. Multiple approaches are thus necessary to distinguish between anthropogenic and natural titanium dioxide nanoparticles. The same holds true for nanoparticles containing other common elements, such as aluminum, silica, calcium, etc. A generic procedure for multi-method analysis is depicted in Figure 9.
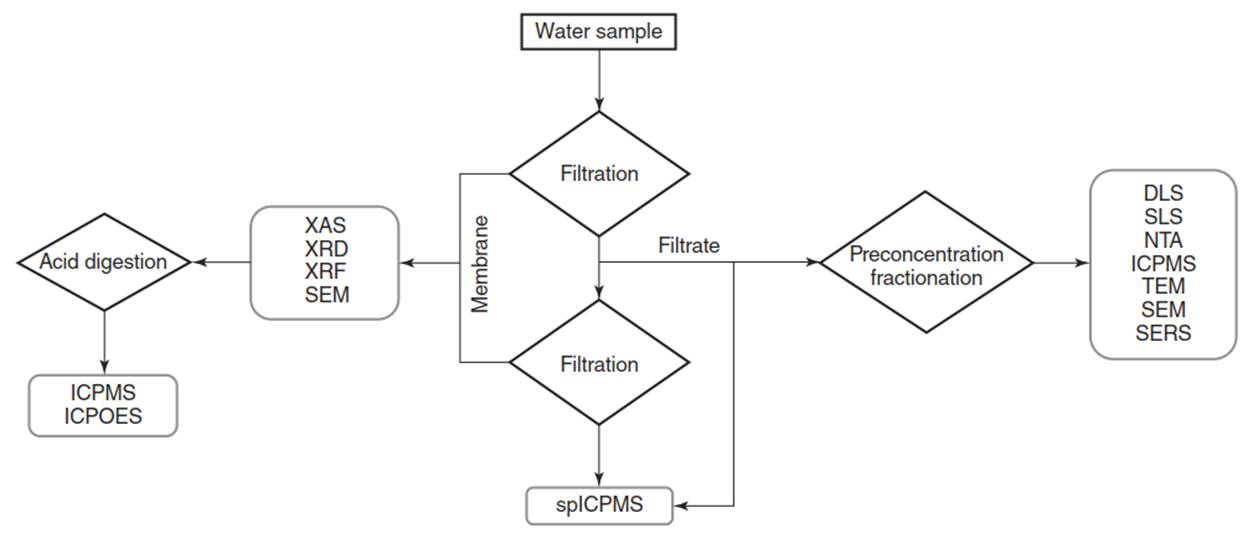
Figure 9. Schematic of an analytical approach for the detection and characterization of nanoparticles in environmental samples
In this approach, large particles and aggregates are removed from the water sample by means of sequential filtration. The pore size of membranes used in filtration can be adjusted to the characteristics of the sample (e.g. high solids concentration, grain size, etc.). Because aggregates containing particles of interest may deposit on the membranes, analysis on the filter membrane is recommended using bulk techniques, such as XAS, XRD, XRF, ICPMS, SEM, etc. The filtrate may then be analyzed with spICPMS and treated with pre-concentration and size fractionation for further analysis with light scattering, mass spectrometry, and electron microscopy.
Date added: 2025-02-13; views: 308;
