The Evolution and Classification of New World Vultures
The history of vultures dates back to at least 50 million years BP, i.e., well into the Cenozoic Era (Rich 1983). Crucially, the grouping into New World and Old World vultures is challenged by fossil evidence, as some New World Vulture ancestral fossils are located in the Old World and some Old World Vulture ancestral fossils are located in the New World. Olsen (1985: 191) notes that 'if the available fossil record is any guide, the so called New World Vultures are definitely misnamed, as their early history is almost completely confined to the Old World'. For example, fossils of Lithornis vulturinus (a possible candidate for Cathartid ancestry) have been dated to 60 million years BP in English sediments. The Genus Lithornis (Lithornis means stone bird in ancient Greek), comprised extinct paleognathous (reptilian) birds from the Upper Paleocene to the Middle Ecoene, which the evidence suggests were good flyers, but some have classified as related to modern tinamous and ratites (the former are mediocre flyers, the latter are flightless).
Houde (1988) describes six species of Lithornis, but the evidence for their validity is disputed. These species are: Lithornis vulturinus (described by Owen (1840) from an Early Eocene fossil in London Clay deposits on the Isle of Sheppey, Kent, and from Denmark (Leona et al. 2005); Lithornis hookeri, described by Harrison (1984) (Mayr 2008); Lithornis nasi described by Harrison (1984); Lithornis celetius from the Bangtail Quarry, Sedan Quadrangle, Park County, Montana, USA, described by Houde (1988); Lithornis promiscuus, from the Early Ecocene Willwood Formation, Clark Quandrangle, Wyoming, USA; and the locationally similar Lithornis celetius and Lithornis plebius described by Houde (1988) from the Bangtail Quarry, Sedan Quadrangle, Park County, Montana, USA.
However, some authors question whether these species are actually Cathartids or Cathartid ancestors. Rich (1983) points out that the evidence for classification is not strong enough. Problems include specimen losses during World War 2, and uncertain classification based on questionable evidence (for example Cracraft and Rich 1972). One classification, by Harrison and Walker (1977) even concluded that Lithornis was neither a Cathartid or a member of Falconiformes, but in the family Threskiornithidae (large wading birds, such as the ibises and the spoonbills).
Apart from the Lithornis group, a number of other discoveries purport to be Cenazoic ancestors of the Cathartids (or Vulturids), but these also are disputed. For example, a species named Teracus littoralis (Milne-Edwards 1867-71) from the early Oligocene of France, was believed to be a Cathartid, but was classified with Incertae Sedis (Olsen 1978a). This is Latin for 'of uncertain placement', for any taxonomic group with unknown or uncertain relationships. Also, bones of a species named Eocathartes robustus were found in Saxony, Germany, but one author stated 'it is almost certainly not a vulturid', but possibly close to the hornbill (Geiseloceros robustus, Lambrecht Olsen 1978a).
Other, possibly stronger examples are Diatropornis ellioti (Milne- Edwards 1892) and Plesiocarthes europaeus (Gaillard), from late Eocene to Oligocene Phosphorites du Quercy in France. Several writers classified these as Vulturids (Cracraft and Rich 1972; Mourer-Chauvire 1982). Another example is Plesiocathartes gallardi of early Miocene (Burdigalian) Spain and another vulture discovered in the early Oligocene of Mongolia (Kurochkin, in Olsen 1985). This fossil evidence may lead to the conclusion that 'New World' vultures had a presence in the Old World. Olsen notes that although the Cathartids were evidently in the Old World as far back as the middle Paleogene, there is no evidence of their presence in the New World until the late Neogene (p. 192).
Other possible Cathartid fossils have been found in the Americas. These were mostly from the Oligocene, Eocene, Pliocene, Pleistocene and Quaternary periods. The pre-Pleistocene fossils are mostly disputed as Cathartid links. Condor-like fossils were dated to the Pliocene (5 million BP) or Pleistocene deposits (2 million BP) (Wink 1995). Olson argued that only Sarcoramphus kernense (L. Miller) from the Pliocene of California and Pliogyps fisheri (Tordoff) from the Pliocene of Kansas are pre-Pleistocene Cathartids in the Americas. Examples of the pre-Pleistocene fossils are Palaeogyps prodomus (Wetmore) and Neocathartes grallator (Wetmore) from Wyoming, but both of these have been described as related to the Bathornithidae, which is now believed to be linked to the modern seriemas of the Order Cariamiformes. There were also Phasmagyps patritus (Wetmore) from Colorado, disputed as the earliest Cathartid (Olson 1985; Stucchi and Emslie 2005). Paracathartes howardae from Wyoming was also argued to be as earliest known Cathartid (Harrison 1978), but this position was challenged by Rich (1983). It was later included in the order Lithornithiformes and family Lithornithidae by Houde (1988).
There were two other extinct, possible Cathartids from the Pleistocene which were unearthed in the Americas. Vultur patruus (Loonberg) of the Pleistocene deposits in Bolivia, has been compared with the current species Andean Condor (Vulturgryphus). It is considered to have a stronger link to the Cathartids than those mentioned above (Fisher 1944; Campbell 1979). Another fossil from the Pleistocene of the New World, which appears more strongly linked to the Cathartids is Breagyps clarki L. Miller from Rancho La Brea, California (Howard 1974; Emslie 1988).
The Golden Age for the Cathartids appears to be from just before to during the Pleistocene and to a lesser extent the Pliocene. These include: Diatropornis (European Vulture), Late Eocene/Early to Middle Oligocene of France; Phasmagyps, Chadronian of Colorado; Cathartidae gen. et sp. indet. (similar in size to the modern Black Vulture) from the Late Oligocene of Mongolia and Middle Pliocene of Argentina and Cuba; Brasilogyps, Late Oligocene-Early Miocene of Brazil; Hadrogyps (American Dwarf Vulture), Middle Miocene of SW North America; Cathartidae gen. et sp. indet. Late Miocene/Early Pliocene of Lee Creek Mine, USA; Pliogyps (Miocene Vulture), Late Miocene-Late Pliocene of S North America; Perugyps (the 'Peruvian Vulture') Pisco, Late Miocene/Early Pliocene of SC Peru; Dryornis (Argentinian Vulture), Early-Late Pliocene of Argentina, which was similar to the modern Genus Vultur; Aizenogyps (South American Vulture), Late Pliocene of SE North America; Breagyps (Long-legged Vulture), Late Pleistocene of SW North America; Geronogyps, Late Pleistocene of Argentina and Peru; Gymnogyps varonai, late Quaternary of Cuba; Wingegyps (Amazonian Vulture), Late Pleistocene of Brazil (Wetmore 1927; Emslie 1988; Suarez 2003, 2004; Alvarenga and Olson 2004; Stucchi 2005).
An important example of vulturine ancestors were the giant teratorns (Greek Tepaxopviq Teratornis, monster bird), the extinct species of the family Teratornithidae (Miller 1925). All the remains for these birds date from the early or late Pleistocene, although some are from the Pliocene (Campbell and Tonni 1983). These giant birds are believed to be linked to the giant megafauna of the period (for example the huge mastodons) and to have died out with their associated mammals (Tambussi and Noriega 1999). There were four genera: Teratornis (species Teratornis merriami, Miller 1909) Aiolornis (species Aiolornis incredibilis, Howard 1952), at least 43 percent larger than Teratornis merriami (Howard 1952); Cathartornis (species Cathartornis gracilis, Miller 1910); and Argentavis (species Argentavis magnificens, dated to the Miocene, Campbell and Toni 1980). Teratorns were the largest flying birds known, with the largest Argentavis magnificens reaching a wingspan of 6-8 m and a weight of 72-79 kg (Campbell and Toni 1980; Campbell and Marcus 1992). Teratornis merriami is the most known of the teratorns, with over one hundred specimens discovered in the La Brea Tar Pits, California and also in southern Nevada, Arizona, and Florida. It was estimated to stand about 75 cm, with a wingspan of around 3.5 to 3.8 m and a weight of 15 kg. The taxonomic history of the Teratorns is highly disputed. The Teratorns are currently placed with the storks (Ciconiidae) and in the order Ciconiiformes (Jollie 1976, 1977; Rea 1983; Olson 1985; Emslie 1988; Chatterjee et al. 2007).
They were first classified with the family Cathartidae, as there appeared to be strong skeletal resemblance between Teratornis merriami (Miller 1909) and the California condor. This was disputed, and arguments arose that classified it as a predator with both raptor and stork attributes (Campbell and Toni 1980, 1982, 1983; Campbell 1995). The taxonomy of T. incredibilis was changed to the Genus Ailornis (Campbell et al. 1999). Miller and Howard (1938: 169) acknowledged that Cathartornis gracilis was 'markedly similar to Teratornis merriami, though it is undoubtedly a distinct species'. This is disputed by Campbell et al. (1999) who argue that the maintenance of Cathartornis as a separate genus is weak.
Several differences between teratorns and modern condors have been gleaned from the fossil remains of Teratornis merriami. The legs of the extinct bird are described as stouter than those of the Andean condor. It is also described as less adapted to the running takeoff, possibly more likely to jump and beat its wings, as the legs were smaller in proportion to the body, with a proportionately smaller stride than in the modern condors (Fisher 1945). Also, it possibly hunted animals including fish (Hertel 1995), as they appear to possess larger stronger bills than the modern condors (Campbell and Tonni 1983).
Large numbers of these birds have been found fossilized in the La Brea Tar Pits. One possible factor for this frequent, curious entrapment was their attraction to dead and dying mammals trapped in the tar (Hertel 1995). The huge birds possibly helped the smaller species (smaller vultures, ravens and eagles) by opening up the carcasses, and would have an advantage over the mammalian scavengers which would be mired in asphalt if they approached. The teratorns may also have attempted fishing from water on the surface of the asphalt.
There are many debates as to the reasons for the extinction of the teratorns. Commonly accepted factors are the climatic shifts at the end of the Ice Age (late Pleistocene/early Holocene). This led to ecological change, extinction of the large mammals, reduced aquatic vertebrate populations, competition from predators for small prey, and from more adaptable smaller vultures (including the condor) for smaller carrion.
Other vulture species also evolved during the Pleistocene. There was a prehistoric species of the Black vulture, Coragyps occidentalis, known as the Pleistocene Black vulture in North and South America, possibly differentiated from the smaller modern Black vulture (Coragyps atratus, Bechstein 1793). Some have argued that it evolved into the modern species by reducing in size after the Ice Age (Fisher 1944; Howard 1962; Steadman 1994; Hertel 1995). If this were the case, the final stage of the evolution may have taken place during the period of human habitation; this is because a fossil bone of the extinct species was discovered in a native midden (or dump for domestic waste) at Five Mile Rapids near Dalles, Oregon, dated about 9000-8000 years BCE (Miller 1957).
There have been disputes in classification of the prehistoric variants, subspecies or ancestral species of the Black vulture. For example, Howard (1968) classified pre-historic birds found in Mexico as Coragyps occidentalis mexicanus, in contrast to the northern sub-species which was classified as C. o. occidentalis (Howard 1968). There was little difference in size between the extinct Mexican birds and modern Black vultures, except that the former had slightly wider and flatter bills, and stouter tarsometatarsus (Arroyo-Cabrales and Johnson 2003). Some writers hold that the prehistoric and modern variants form a continuum, and include C. occidentalis in C. atratus (Steadman 1994).
Concerning the current New World vulture species, there are seven species and five genera currently recognised: Coragyps, Cathartes, Gymnogyps, Sarcoramphus, and Vultur. The characteristics of New World vultures are featherless heads and necks (Zim et al. 2001); long, broad wings and a stiff tail suitable for soaring (Ryser and Ryser 1985); clawed but weak feet (Krabbe and Fjeldsa 1990), long front, slightly webbed toes (Feduccia 1999); the lack of a syrinx or voice box, limiting their vocalizations to hisses or grunts (Kemp and Newton 2003; Howell and Webb (1995); a slightly hooked, comparatively weak bill, compared to other raptors (Ryser and Ryser 1985; Krabbe and Fjeldsa 1990); oval, perforate nostrils not divided by a septum, i.e., one can see through the bill by the nostrils (Terres 1991; Allaby 1992); the absence of the bony brow of raptors (Terres 1991) (see Fig. 4.1); and the storks' habit of urohidrosis, defecating on their legs for evaporative cooling. Cathartes is regarded as the only genera that is not montypic; where monotypic refers to the possession of only one immediately subordinate taxon (for example a subgenera). Therefore, the Genus Cathartes has three species, while the rest have only one species each (Myers et al. 2008).
Regarding the classification of the New World vultures, there have been disparate views since the late 19th century. Initially, these were placed in their own family within the Falconiformes (Sibley and Ahlquist (1991). The conflicting views may be classified into three: (1) those that hold that the New World vultures are related to the storks and belong in the Ciconiiformes; (2) those that hold that they should be in the Falconiformes; and (3) those that hold that they should be in their own Order. Earlier studies were based on morphology, but in the late twentieth century DNA and other biochemical based studies were also published.
Brown and Amadon (1968: 17) recognised four main groups of vultures, 'singling out the New World vultures as the most likely to be unrelated.' A close phylogenetic (multi-ancestral) relationship with storks was suggested (Garrod 1873; Ligon 1967; Konig 1982; Rea 1983). Garrod (1873) placed Cathartids between storks and herons, due to thigh muscle form. Goodchild (1886) used a study of secondary wing coverts (small wing feathers) to determine that the Cathartids were similar to Ciconiiformes, Procellariformes and four families of Pelicaniformes, but different from the Accipitriformes (see also Beddard (1889, 1898). Sharpe (1891) used the details of intestinal coil patterns to place the Cathartids with the Ciconiiformes, Procellariformes and Pelicaniformes. Hudson (1948) used the pelvic musculature of the Falconiformes, and concluded that the Cathartids, Accipitrids and Sagittarids evolved as different lineages. Friedmann (1950) stated that 'the exact relationship of the Cathartidae, is however somewhat complex. It has been fairly clearly demonstrated that they are not very distantly related to the Ciconiiformes, Pelicaniformes and Procellariformes.' Ligon (1967) used evidence from osteology, natal plumage, myology, patagial tendons and syringeal structure, to combine the Cathartids and Ciconiiformes; placing the herons by themselves in the Order Ardeiformes.
Jollie (1976) used pterylography (the study of feather arrangement), osteology (the study of bone structure) and myology (the study of muscle structure) to conclude that the Falconiformes included four phylogenetically unrelated groups; an important factor for this decision was the physical distinctions of the New and Old World vultures. The distinctions between these groups concerned the lack of the raptor grasping foot and a syrinx in the New World vultures, that distanced these birds from raptors. Factors that related the Cathartids to the storks were the defecation on the legs, wing spreading, bill clapping and bill interlocking before and during copulation, the inflation of neck sacs and gender—neutral incubation (see also Konig 1982; Rea 1983; Sielbold and Helbig 1995).
Fisher (1955) used only morphological evidence to distinguish the New World vultures from the Old World vultures. Basically, the skulls of the cathartid vultures 'may be distinguished from the skulls of the other Falconiformes' in that: 'the external nares are perforated; the rostral area of the skull is elongated (except in Sarcoramphus); an imperfect frontonasal hinge is present; the lachrymals are completely fused to the frontals and are directed downward; the premaxilla is highly vaulted; the opisthotic processes are extremely long; the articular process of the squamosal is weak or absent; the sphenoidal rostrum is excavated in front of the basitemporal plate; the bones within the olfactory chamber are more completely ossified; the zygomatic arch is split anteriorly; and the skull is indirectly desmognathous' (ibid.).
For an explanation of these terms, the nares are nostrils. In New World vultures, these are perforated; it is as if a hole has been drilled through the bill at right angles to the direction of the beak. In the Accipitrid vultures, the nasal passage is divided by a septum, composed of cartilage and bone. The rostral area of the skull refers to the part of the skull connected to the bill, as the rostrum refers to the bill itself. New World vultures (with the exception of the king vulture), have more elongated heads, compared with the Old World vultures. The former also differ from the long heads of the Hooded and Egyptian vultures, as in these two small Old World vultures the skull is not long, only the bill is long (see Fig. 2.9). The frontonasal hinge refers to the connection between the bill and the frontal and nasal bones, a bony plate composed of extensions of the nasal, frontal and premaxillary bones. The frontal bones comprise the front of the cranial cavity and forms the forehead. The nasal bones refer to the bones that together form the bridge of the nose. The lachrymals refer to the glands that produce tears, which are fused to the frontal bones in the New World vultures. The opisthotic bone, along with the prootic and the epiotic, forms the petrosal or periotic bone of the head, behind the ear (Coues 1883). The articular process of the squamosal refers to the articulation with the quadrate bone of the lower jaw. The sphenoidal rostrum and the basitemporal plate are both parts of the skull. The olfactory is the cavity inside the nose, used for respiration and in some cases smell. The zygomatic arch is the cheekbone on the side of the skull (Olsen and Joseph 2011).
Fisher (1944) also pointed out differences between the skull of the King vulture and some of the other Cathartids. In the former, the angle of the bill forms an acute angle of about 30°, while in Cathartes it is 12 to 18°. This gives the skull and bill of the King vulture a 'definite predatory aspect in contrast to the weak-billed, flat-topped condition found in the other cathartids' (ibid. 278). This is also argued to indicate a stronger bill in the King vulture, justifying its status as a tearer. The lower mandible is also strongest in the King vulture, and progressively weaker in the Andean condor, the Cathartes vultures and the Black vulture. See Fig. 4.3 for the profiles of some of the Cathartid heads. The King vulture can open its bill more widely than can the other Cathartids. The Black vulture, having a longer bill than the rest, also has a wider gape. It is noted that
'ability to open the bill more widely may be an adaptation in two ways. In grasping live, struggling prey a widely opened bill is an aid to securing and maintaining a strong hold. In the case of Sarcoramphus it probably aids in this manner as well as in enabling larger chunks of carrion to be swallowed. The latter adaptation is perhaps the more important in Coragyps, but here again the aid in grasping prey may be significant since McIlenney (1939) has shown that Black Vultures attacking in groups kill skunks, opossums and other small mammals' (ibid. 285).
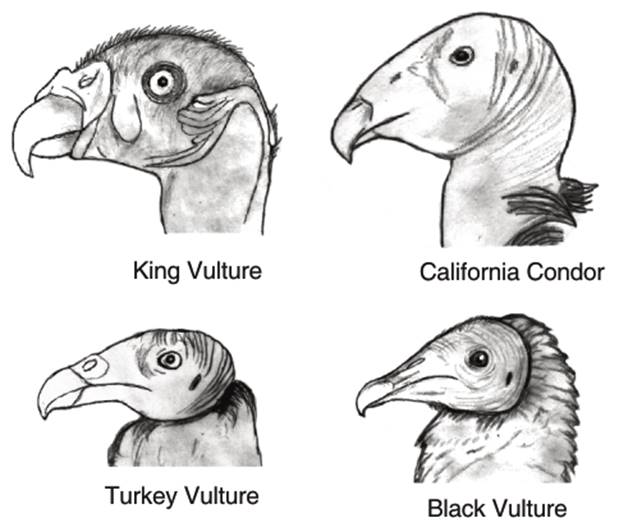
Fig. 4.3. The Heads and Bills of some Cathartids
The Cathartids were nevertheless included within the Falconiformes, or Acciptriformes until the 1990s. Studies supportive of this view include those of Wetmore (1960), Brown and Amadon (1968), Stresemann and Amadon (1979), Cracraft (1981) and Sielbold and Helbig (1995). Later writers during the 1990s however continued to acknowledge the possiblity of a Cathartid and stork link and convergent evolution (Konig 1982; Sibley and Ahlquist 1990).
The raptor placement was challenged in favor of a closer relation with storks during the late 20th century, referring to behavioral, karyotype, molecular and morphological information (Ligon 1967; de Boer 1975; Konig 1982; Rea 1983; Seibold et al. 1993, 1995; Wink 1994; Wink et al. 1993a,b, 1996). The karyotypes studies are based on the number and appearance of chromosomes in the nucleus of a eukaryotic cell, and/or the set of chromosomes in a species. Behavioral traits include defecation on the legs for cooling purposes, this being shared with storks. Morphological issues concern the composition of the uropygial gland secretions, leg and pelvic muscle anatomy, and the distribution of feather lanes (Wink 1995).
During this period, the placement with or near the storks was also challenged using both molecular and morphological evidence (Griffiths 1994; Cracraft et al. 2004; Ericson et al. 2006; Gibb et al. 2007; Slack et al. 2007; Livezey and Zusi 2007; Hackett et al. 2008). These new studies used marker genes, the better to describe a 'more precise phylogenetic reconstruction of relationships within and between genera, subfamilies and families of birds' (Wink 1995: 868; see also Avise 1994; Sibley 1994) because marker genes can be easily amplified by PCR and sequenced (Kocher et al. 1989; Hillis and Moritz 1990; Taberlet et al. 1992; Edwards and Cracraft 1993; Kornegay et al. 1993; Hedges and Sibley 1994; Meyer 1994). More recent studies used deoxyribonucleic acid (DNA) (Sibley and Ahlquist 1990; Avise 1994; Sibley 1994). DNA is a molecule that contains the codes of the genetic instructions for the development and functioning of living organisms. These studies use the nucleotide sequence of the mitochondrial cytochrome b (cyt b) and are able to record phylogenetic events (evolutionary changes in organisms) as far as the last 20 million years (Seibold et al. 1993, 1995; Wink 1994; Wink et al. 1993a,b, 1996). This technique has been justified in some studies from the 1990s (Edwards et al. 1991; Richman and Price 1992; Helm-Bychowsky and Cracraft 1993; Kocher et al. 1989; Meyer 1994; Ta berlet et al. 1992; Heidrich and Wink 1994; Heidrich et al. 1995; Wink 1994; Wink et al. 1993a,b, 1994, 1996; Seibold et al. 1993, 1995).
The results of these new studies were not conclusive, as some found that New World vultures were a subfamily of storks (Ligon 1967; Sibley and Monroe 1990), others found they were more closely related to storks than to raptors, while other results showed they were not related to either storks or raptors (Rea 1983; Jacob 1983).The placement as a subfamily of storks was criticized as an oversimplification (Griffiths 1994; Fain and Houde 2004). An early DNA sequence study in this direction was based on erroneous data and was subsequently retracted (Avise 1994; Cracraft et al. 2004; Gibb et al. 2007; Brown and Midell 2009).
Other studies did not place them with storks, but nevertheless found a close relationship, possibly closer than with raptors. Avise et al. (1994) looked at the nucleotide sequences of cyt b from New World vultures in relation to storks and other members of the Ciconiiformes, and found these vultures appear to be more related to storks than to Old World vultures. The relation to storks was supported by many studies (Konig 1982; Rea 1983; Sibley and Ahlquist 1990; Avise et al. 1994; Seibold 1994; Seibold et al. 1993, 1995). However, some studies found only weak links with storks and Accipitrids. Siebold and Helbig (1995) tested the phylogeny of 11 species of Old World vultures, three species of New World vultures and related species, using 1026 nucleotides of the mitrochondrial cytochrome b gene. New World vultures were found to be unrelated to the Falconiformes, but the evidence of a closer link to storks than to Falconiformes was insufficient.
Wink (1995: 880) showed an interesting result; the Cathartidae are not closely related to the storks, but also not related to the Accipitridae. This author noted that despite morphological data and DNA-DNA hybridisation linking New World vultures and storks ancestrally (Garrod 1873; Ligon 1967; Konig 1982; Rea 1983; Sibley and Ahlquist 1990), the analysis showed separation between storks (Ciconia, Leptoptilos and Mycteria) and New World vultures, and similar genetic distances were found between the New World vultures and Old World vultures, condors and storks, and storks and Ac cipitridae. The close relationship of cathartid vultures with storks was thus unlikely. The results also did not support a close relation between the stork Jabiru mycteria and the Black vulture as had been indicated by Avise et al. (1994).
Wink's study was described as more comprehensive as it combined both sets of sequence data for 6 of the 7 New World vultures and 11 of 15 Old World vultures, and looked at the phylogenetic relations within Old World vultures, between New and Old World vultures and also the relations between both groups and other Accipitridae raptors, and also with the Ciconiiformes. The results also justified the classification of carthartid vultures into several monotypic genera (except for Cathartes with 3 species) (Wink 1995). Findings included a closer link between the California condor and Black vulture than between the latter and the Andean condor, and links between the Turkey vulture and the Yellow-headed vulture (Wink 1995). The first result is less obvious than the second, using morphological data.
A new, independent Order of New World vultures, Cathartiformes was also mooted (Ericson et al. 2006). This was challenged in 2007 by the American Ornithologists' Union Committee on Classification and Nomenclature and North American checklist, which placed the Cathartidae back into the Falconiformes (Banks et al. 2007), but left open the possibility of error: i.e., it is 'probably misplaced in the current phylogenetic listing but for which data indicating proper placement are not yet available' (American Ornithological Union 2009). Nevertheless, the AOU's draft South American checklist put the Cathartidae in their own order, Cathartiformes (Remsen et al. 2007, 2008). In the AOU's 2010 North American checklist, the Cathartidae family was placed in the Order Ciconiiformes. Further challenges arose, as a recent multi-locus DNA study indicates that New World vultures are related to the raptors, with the exception of the true falcons Falconidae (Hackett et al. 2008). In this analysis, the New World vultures should rather be part of a new order Accipitriformes (American Ornithologists' Union 2010).
Date added: 2025-04-29; views: 285;
