Analogies, Homologies, and Homoplasies. A Framework for Comparisons
As noted in the Preface, a principal focus of this book is to develop analogies between the ecology of microorganisms and macroorganisms. In this context, ‘analogy’ is broadly construed and used in its popular connotation to refer to parallels, similarities, or resemblances of interpretative value in contrasting strategies or life histories, without implying common ancestry. The word ‘analogy’ also can be used more specifically in a scientific context (though rarely so in this book) for comparisons of similar traits of independent evolutionary origin, i.e., those that have arisen by parallel evolution or convergence. Thus, the wing of an insect and the wing of a bird are analogous. Such traits are said to be homoplasies (Givnish and Sytsma 1997).
In contrast, homologies imply similarity due to common ancestry, and features can be homologous even if they differ functionally, as in the forelimbs of bats, porpoises, and humans. Homologies can be further subdivided as primitive or derived in ancestral origin. For instance, since all mammals are vertebrates, they have a backbone, which is considered a primitive characteristic manifested by the ancestor common to the entire vertebrate lineage, including animals such as the lampreys, fish, and reptiles. Possession of hair, however, is unique to the mammal clade. Since hair is a trait that arose in an ancestor more recent than the ancestral vertebrate, it is a derived character.
A Framework for Comparisons. Competing Demands, Trade-Offs, and Resource Allocation.All organisms can be viewed simply as input/output systems (Fig. 1.2; Pianka 1976, 2000). Each acquires some kind of resource (energy and materials) as input. Progeny are the output. Under natural selection, each creature will tend to allocate limited inputs optimally among the competing demands of growth, maintenance, and reproduction (Gadgil and Bossert 1970; Abrahamson and Caswell 1982; Karasov and Martinez del Rio 2007). To the extent to which each of these activities is clearly an alternative (a debatable point, below), an increase in one necessarily results in a decrease in one or both of the others. As developed below and in later chapters, this allocation principle entails trade-offs because there are finite resources and finite time to meet these competing needs.
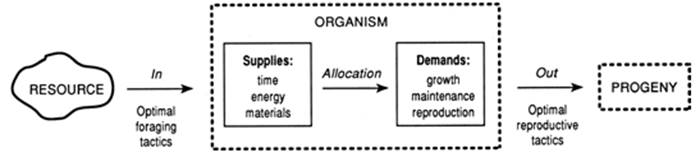
Fig. 1.2. The organism as an input/output system. Redrawn and revised figure based on Pianka (1976) originally from American Zoologist by permission of Eric Pianka and Oxford University Press ©1976
As is the case for all models, the input/output concept is of course a simplification. The evidence for allocation patterns in particular taxa is mixed and ‘alternative’ demands when examined in detail are not as clear-cut as appears intuitively and superficially. Nevertheless, the generality holds and our attention here is not as to whether it is invariably correct but that it provides a good vehicle for comparing how microorganisms and macroorganisms live. Clearly, the focus in the model is on the major life history attributes. It is worthwhile to consider briefly what is not represented explicitly. The most glaringly obvious yet perhaps the most easily overlooked point is that it centers on the individual operating as a singular unit, whereas probably all organisms are functionally collaborative entities through various mutualisms. A classic case is the reliance of macroorganisms on microorganisms for services (e.g., Chap. 3 and McFall-Ngai et al. 2013).
A ‘hidden’ output in the model is metabolic products, typically invisible (e.g., oxygen in the case of plants; various secretions in the case of animals; extracellular polysaccharides and organic acids for microbes), but are not unimportant, especially in the microbial world! Responses of the individual to predation or competition, while not recognized explicitly, would be reflected in the model by altered biomass allocation patterns and in the output term as altered number of progeny. Foraging tactics involve the obvious maneuvers such as predators hunting in packs rather than alone, with the likely microbial equivalent being the primitively multicellular microbes such as Myxococcus, which “feeds as a pack of microbial wolves, with each cell benefiting from the enzymes secreted by its mates” (Kaiser 1986).
Other foraging devices may include protecting a resource by various secondary compounds such as antibiotics, mycotoxins, or staling products (e.g., Janzen 1977a; Burkepile et al. 2006), or directly poisoning competitors. In the same vein, attracting mates or pollinators, or defending a breeding territory from competitors or predators, are a part of optimal reproductive tactics. In other words, fitness can be increased by high acquisition and efficient allocation of one’s own resources, but also by interfering with those same activities of a competitor.
Fitness or ‘success’ is measured by the progeny or output over time, but it is more than just progeny numbers: By definition, the fittest individuals leave the most descendants as alluded to above. In population genetics terms, the number of descendants amounts to the proportion of the individual’s genes left in the population gene pool (Pianka 1976). Given the diversity of life forms, what constitutes an individual is not clear and need not be in the context of the overall model. Some connotations of ‘individual’ are discussed below in 7Sect. 1.6 and at length in Chap. 5. In addition to being transient, success is thus assessed in a relativeway, against other members of the population. The issue of the fitness or competency of the offspring, that is, the likelihood that they will go on to reproduce and perpetuate the lineage, and thereby become descendants, is not specified in the general model.
Acquisition (input) Three general points will be made here concerning resources as a prelude to subsequent chapters (Fig. 1.2). First, a basis for grouping all life forms is how they meet requirements for organic carbon compounds and energy for growth, maintenance, and reproduction (Chap. 3). With respect to carbon, organisms are either autotrophic (literally, ‘self-feeders’, or CO2 fixers) or heterotrophic (‘fed from others' i.e., they use organic forms of carbon). With respect to energy source, organisms are either phototrophic (source is light) or chemotrophic (source is chemical substances). If inorganic chemicals are oxidized for energy, the organisms are said to be lithotrophic, whereas organic chemicals are oxidized by organotrophs.
These characteristics set very broad limits on what organisms can do and consequently where they are found, or at least where they can grow actively to competitive advantage. One example pertinent to each requirement will suffice to make the point. The need for sunlight restricts most aquatic plants to relatively shallow depths (photic zone) of oceans and most lakes. The distribution of those bacteria that obtain their energy by oxidizing specific inorganic compounds such as sulfur or iron (lithotrophs) tends to mirror that of the particular deposit. Members of the genus Sulfolobus, for example, live mainly in hot springs and in other geothermal habitats rich in sulfur.
Assignment of all organisms to broad resource categories also establishes the well-known trophic structure of communities, which is informative in terms of energy flow and nutrient cycling. Since a high percentage of energy is dissipated by organisms in maintenance (and as heat and motion), not only is it probable that the number of links is set ultimately by energy losses at each, but the density of individuals and total biomass typically decrease at each successive trophic level (for caveats and details, see Chap. 3).
The second generality concerns how resources are presented to the organism. All organisms must contend with variation in the distribution and abundance of resources. Environments can be characterized with respect to resource availability (and other attributes) from the organism’s viewpoint as relatively heterogeneous (discontinuous, patchy) or comparatively homogeneous (uniform). Patches are dynamic in that they vary in characteristics such as content, size, time, and spatial orientation.
The idea of environmental grain (Chap. 7; see also Levins 1968; Jasmin and Kassen 2007a) relates the size of a resource patch to the size of the individual and to the space within which the organism is active. Coarse-grained environments are sufficiently large that the individual either comes by fate to spend its entire life in a patch (for instance, when deposited there as a seed or spore) or chooses among them (for example, a millipede living only within a rotten log). Where the patches are so small that they appear uniform to the individual, or when the individual encounters many states of the heterogeneity during its lifetime, the environment is fine-grained.
The larger relative to the patch size, the more mobile, or the longer lived the organism, the more likely it is to ‘see’ its surroundings as fine-grained. Plants in a field would appear fine-grained to humans or a grazing deer, but coarse-grained to an insect larva and immensely so, even at the level of the individual leaf, to a bacterium. A butterfly flits across several hundred square meters of a field in fine-grained fashion, while a slug, sliding slowly on its mucus trail across only a few meters of that same field, experiences its surroundings as coarse-grained.
Over its life span of several hours or days, a bacterial or yeast cell on the skin of an animal or in a plant exudate confronts a coarse-grained environment, whereas typical changes on this order of time would appear relatively fine-grained to the host on which that microbe finds itself. In contrast to unitary organisms such as deer or humans, in modular organisms (see below), the genetic individual commonly samples many environments concurrently. Sporadic and cyclic changes in resource availability (and other environmental characteristics) have immense influence on life history features such as dispersal, migration, and dormancy (Chaps. 6 and 7).
Third is the issue of efficiency of resource utilization. This has been expressed in optimal foraging theory (OFT) and appears explicitly as part of Fig. 1.2. OFT was developed originally in a particular context by animal ecologists (Chap. 5 in Pianka 2000). When the concept is broadly construed and used in conjunction with optimal digestion theory (Chap. 3), however, it is easy to see analogies and implications for all organisms. The basis for the theory is that there are both benefits, such as matter and energy, and costs, such as exposure to predators or parasites, or time and energy diverted from other activities such as reproduction, associated with foraging. Under natural selection, organisms presumably have evolved some sort of efficient ‘foraging strategy’ that recognizes both the benefits from and costs directly associated with resource acquisition, as well as implications to other competing demands. The use of optimal foraging models as one case in point for optimization models in general should be employed not as an attempt to assume or prove that organisms are ‘optimal’, but to understand how particular adaptations, trade-offs, and constraints shape evolution (Maynard Smith 1978a; Parker and Maynard Smith 1990; for broader insights on the utility of optimality models, see Stearns 1992).
To a species of bird, for example, optimal foraging might concern net energy gained or lost in the catching of insects of various sizes at various distances from its perch. How small a prey item is too small, or how far away is too far, to make a trip worthwhile? Under what circumstances would it be better to adopt a ‘sit-and-wait’ tactic as opposed to actively searching for prey? A microbial analogue might be whether it is better to synthesize a key metabolite, such as ATP, quickly if wastefully (lower yield), or implement pathways that produce substantially more product but much more slowly (7Chap. 3 and Pfeiffer et al. 2001). Plants ‘forage’ from a more or less fixed location in the sense that they display leaf canopies for photosynthesis and root systems to collect water and nutrients. How branch, leaf, and root architecture has evolved to meet these needs and in the face of competitive and predation pressure is of much interest (7Chap. 5; see also Horn 1971; Bell 1984; Givnish 1986). Analogously, sedentary filter-feeding invertebrates (e.g., bivalves, brachiopods, ectoprocts, and phoronids), and both attached and motile microbes ‘forage’ (e.g., van Gestel et al. 2015), although in many cases foraging may entail a sit-and-wait strategy (quiescent spores of a root-infecting fungus in soil; sessile, stalked bacteria of the genus Caulobacter).
The form of living things as it pertains to resource utilization can be generalized further. Sessile modular organisms such as plants, corals, bryozoans, fungi, and clonal ascidians (as opposed to unitary organisms; 7Chap. 5) capture space and other resources by a branching habit of growth. In so doing they create ‘resource depletion zones’ (Harper et al. 1986). These zones might represent, for instance, a shaded area on understory leaves resulting from interception of light by a tree, or a volume of soil from which nutrients had been partially removed. Many biological systems exhibit dichotomous branching. Why? A major challenge to branching, modular organisms regardless of size is development of a branching pattern to effectively capture resources. As detailed in Chap. 5, a continuum can be visualized between two extreme growth forms, phalanx (closely packed branches; resource site densely occupied) and guerrilla (infrequent branching; rapid extension; much unoccupied intervening space) (Lovett-Doust 1981a, b). Thus, there is a correspondence between optimal foraging theory and optimal branching strategy. The issue is not straightforward because selection for one activity or function cannot proceed independently of other selection pressures. The challenge is visually dramatic with respect to morphology and hence has been emphasized in the case of branching and resource harvesting.
Reproduction (output) Having acquired resources, the organism must then allocate them among the competing needs of growth, maintenance, and reproduction. To delay reproduction will be advantageous only if more descendants are ultimately contributed to future generations. For example, formation of large, perennial fruiting structures by certain of the basidiomycete fungi is undoubtedly expensive in diverted materials, time, and energy, but offers the multiple advantages of an enhanced platform for sexual recombination and widespread aerial dispersal of millions of propagules.
Use of time and energy by organisms tends to vary seasonally (Chap. 6) as is apparent to anyone who has collected mushrooms in the autumn or followed the varying activities of birds over a few months. Reproductive activities are typically well synchronized to periods of the year. For many animals the breeding season coincides with environmental conditions favorable for survival of parents and offspring. Birds become highly territorial and nest in the spring in temperate regions; parasites are remarkably well coordinated to the behavior or phenological development of their hosts. The fact that sexuality in algae and fungi is frequently triggered by environmental adversity is not inconsistent with the above generality, because the resulting zygote is typically enclosed in a thick-walled, resistant, dormant structure functionally analogous to seeds in plants. While maintenance and repair activities are usually ongoing at the cellular level (Kirkwood 1981, 2005), they are especially apparent at the level of the individual as stages of rest. In one form, maintenance is recognizable in the circadian sleep cycle typical of higher animals; in another, as seasonal inactivity (winter hibernation; plant dormancy). Although obviously essential for survival, and the preparations necessary for resuming activity, maintenance represents lost time, energy, and materials to the extent that limited resources are diverted from reproduction.
So-called ‘optimal reproductive tactics’ (Fig. 1.2) seemingly have evolved by natural selection acting on organisms, which as a result have improved long-term reproductive success. As for foraging, the issue is again one of relative allocation: resources and time assigned to reproduction are diverted from other activities. And, what is allocated to reproduction may be invested in various ways. For instance, a partial suite of either/or reproductive ‘choices’ includes whether to: (i) engage in only one round of reproduction (semelparity) or several (iteroparity) during a lifetime; in the latter case a further question is how many episodes to have; (ii) have one mate or several or none (clonal or asexual reproduction); (iii) reproduce early or late in the life cycle (or season); (iv) produce few large, highly endowed or ‘competent’ progeny or many small, relatively ‘incompetent’ progeny; related to this frequently is the length of gestation; (v) reproduce locally or risk migration to new breeding grounds; (vi) maintain separate sexes (sexual dimorphism; dioecy; heterothallism) or unite sexual function within one individual (hermaphrodism; monoecy; homothallism); related to this is the capacity to vary the sex ratio and the even larger question of why there should be two, rather than some other number of sexes; (vii) disperse progeny widely or concentrate offspring near the parents. Regardless of the countless variations on the theme of reproduction, the ultimate test of a strategy is whether it results not in more progeny, but more descendants.
Longevity is tied closely to reproduction. In evolutionary terms, senescence is expected to occur wherever the reproductive value of the individual declines with time (for terminology and details, see Chap. 6). Under relatively stable environmental conditions and where the reproductive value of the individual increases with age, as it typically does for modular (unlike unitary) organisms, senescence should be delayed or not occur (Harper et al. 1986). Under these circumstances, the genetic individual (Sect. 1.6) lives for an indefinite time and there is the potential for exponential increase in offspring, especially where the individual is also clonal (Chap. 6).
The ecological issues noted above and summarized in Fig. 1.2 are developed in subsequent chapters as follows: Since variation provides the raw material for evolution, the stage is set in Chap. 2 with an overview of the genetics and mechanisms of genetic variation of the larger as opposed to the minute organisms. In Chap. 3 we take up the supply side of Fig. 1.2 and consider the kinds of resources different organisms use, and how they acquire and allocate them in the face of competing demands. This leads to a consideration of how size (Chap. 4) and growth form (Chap. 5) influence foraging and reproduction. Chapter 6 concerns the origins and molding of the life cycle, in essence how the developmental play is acted out under the direction of the genes. Senescence is considered at some length, including the controversial issue of whether it is inevitable. The role of the environment in shaping the plot (life history features) is discussed in Chap. 7. Some general conclusions are summarized and perspectives are advanced in Chap. 8.
Date added: 2025-06-15; views: 219;
