Asexual Reproduction in Organisms: Evolutionary Implications, Clonal Diversity, and Genetic Consequences
While most if not all extant organisms reproduce sexually, some are facultatively and others apparently obligately asexual. Asexuality is discussed here at some length because of its significant evolutionary implications and to set the stage for our later discussion of what constitutes a genetic individual. There are numerous variations on the theme of asexuality, each with its own terminology that is frequently inconsistent across disciplines (Normark et al. 2003; Jackson et al. 1985; Schon et al. 2009; Tibayrenc and Ayala 2012). The prevalent modes are: (i) apomixis, which strictly means that female progeny arise mitotically from unfertilized eggs. Its usage, however, is varied and is often equated with agamospermy or parthenogenesis, or alternatively equated with all forms of asexual reproduction; (ii) vegetative growth, wherein new individuals arise by fission or budding—as is exemplified by bacteria and yeasts, respectively; or fragmentation as in some plants and animals; and (iii) automixis, where eggs are produced meiotically but the meiotic products refuse. Clearly, the various modes can have somewhat different genetic consequences.
The entire set of individuals that descends exclusively (i.e., asexually) from a common ancestor is called a clone. As Milkman points out (1996) ‘exclusive’ means that all the genetic material in all the descendants originates with the common ancestor. The exact meaning of ‘clone’ varies by discipline and microbiologists usually have a more specific and practical context in mind than do botanists or zoologists; also, usage in microbiology is complicated by inconsistent semantics of multiple terms such as serotype, ecotype, sequence type, lineage, strain, and clonal cluster (for microbial examples, see 0rskov and 0rskov 1983; Anderson and Kohn 1995; Spratt and Maiden 1999; Henriques-Normark 2008; Tibayrenc and Ayala 2012, 2015; for macroorganisms, see Jackson et al. 1985; Hughes 1989). As one operational example from medical microbiology pertaining to Staphylococcus aureus genotyping, isolates that have identical nucleotide sequences at all of seven housekeeping genes are considered to belong to the same ‘clone’ and receive a unique ‘sequence type’. Those that are identical at five or more of the loci are known as a ‘clonal complex’ (Chambers and DeLeo 2009).
The word ‘clone’, used as a noun or more commonly today as a verb, has undergone considerable variation in meaning since its first application apparently in plant breeding in the early 1900s. Regardless of its current application in many subdivisions of biology, each with its own semantics, one should not infer that all members of a clone are necessarily genetically identical, despite that implication being drawn by some authors. While in the early stages of growth and depending on the mode of asexual reproduction (see above), all clonal members may be essentially identical, they diverge over time due to somatic mutation. The nested relationship of progressively diverging clones, most easily visualized for bacteria, is nicely illustrated by Milkman (1996; see also Spratt and Maiden 1999) (Fig. 2.7).
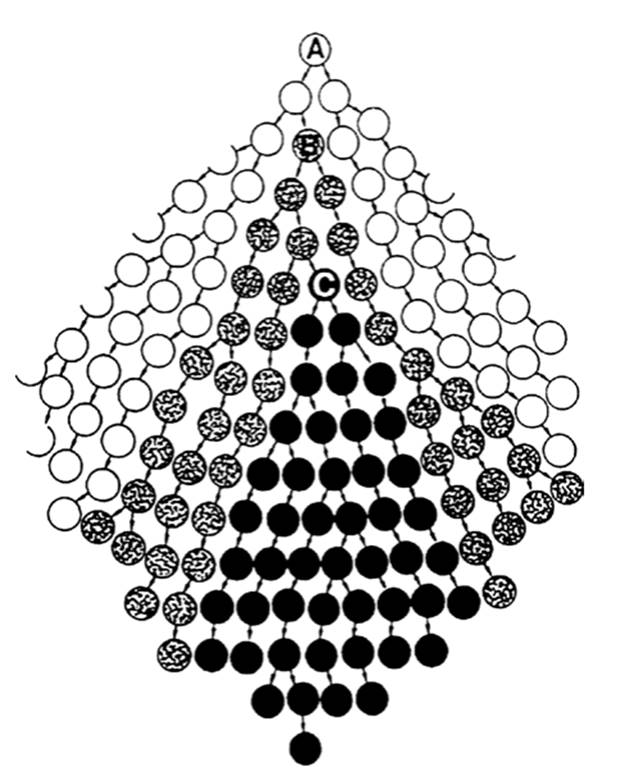
Fig. 2.7. Clonality as a hierarchical or nested relationship with clones originating exclusively from a single common ancestor. This is most easily visualized as a progressively diverging series of bacterial cells dividing by fission, but is in principle applicable to all clonal organisms. Clone A is the oldest and progenitor of sub-clone B and eventually and indirectly of sub-clone C; B arises from A by (somatic) mutation and C diverges from B by further mutation. All three clones are closely related and ultimately traceable to cell A, but each has its own age and genetic structure. The process continues indefinitely. See text for complications and caveats. From Milkman (1996); reproduced by permission of ASM Press, Washington, DC ©1996
However, some authors have defined clones more broadly. Writing mainly with respect to microorganisms, Tibayrenc and Ayala (2012, p. E3305; see also Spratt et al. 2001) argue that ... “clonality does not mean the total absence of recombination, but that it is too rare to break the prevalent pattern of clonal population structure.” Their key defining criteria for clonality are: (i) strong linkage disequilibrium together with (ii) clear phylogenetic signal. This would constitute “predominant clonal evolution” (Tibayrenc and Ayala 2015). Unfortunately, it is often not a simple matter to differentiate between episodes of mutation and recombination, either in microorganisms (Bobay et al. 2015) or macroorganisms (Ally et al. 2008). In prokaryotes, genotypic and phenotypic clonal divergence can occur rapidly (within hours to days) in culture, whether in heterogeneous (Rainey and Travisano 1998) or constant (chemostat; Maharjan et al. 2006) conditions (for general remarks, see Bobay et al. 2015).
Thus it is the case both in vitro and in vivo that multiple clonal sub-lineages occur, each carrying at least one and likely many mutations. These subpopulations or ‘mutational cohorts’ compete in a phenomenon commonly referred to as ‘clonal interference’ (e.g., Williams 1975), and the population is not necessarily purged of variation by selective sweeps of a clearly superior line as once thought (Lang et al. 2013). Because of their shorter generation times and relatively much larger population sizes, microbial clones diversify faster in absolute time than clones of macroorganisms such as aphids or aspen trees.
In phylogenetic terms, lineages of asexual eukaryotes tend to be scattered among branches of their sexual relatives near the tips of phylogenetic trees. This implies that, in general, asexuals have arisen relatively recently and are short-lived in evolutionary time, without having had the opportunity to diversify to a high taxonomic rank (Butlin 2002). Phylogenetic evidence, along with molecular genetics data attesting to the ancient and complex nature of the sexual process, suggest that asexuals have arisen sporadically from sexuals, rather than the other way round (Otto and Lenormand 2002; Rice 2002).
Why asexuals generally do not persist is hotly debated. Stanley (1975) argued that most evolutionary change arises from speciation events and that asexual macroorganisms generally could not speciate rapidly enough over evolutionary and geological time to avoid extinction, thus accounting for the paucity of asexual clades. Another reason may be the relentless accumulation of deleterious mutations in a finite asexual population (‘Muller’s ratchet’ noted earlier; Bell 1988; Barton et al. 2007). The rate at which the ratchet turns and its impact are subject to several assumptions. Depending on which ones are invoked, the theory actually predicts that asexuals would be eliminated quickly, slowly, or not at all (Normark et al. 2003). The common condition of polyploidy in asexuals has been interpreted as possibly insulating the organism from the deleterious effect of accumulating mutations (Otto and Whitton 2000; Pawlowska and Taylor 2004).
There have been numerous claims for ancient asexuals, occasionally referred to as “ancient asexual scandals” because, if true, the examples contradict conventional wisdom that asexual lineages cannot persist long (Muller 1964; Maynard Smith 1986; Bell 1988; Normark et al. 2003). As emphasized by Judson and Normark (1996), such records must meet all three components inherent in the term ‘ancient asexual group’ namely that the lineage is: (i) descended from a common ancestral group (i.e., is monophyletic); (ii) ‘ancient,’ defined subjectively but commonly taken to be on a scale of geological time, an order of magnitude of millions or tens of millions of years; (iii) primitively asexual, i.e., that the group has remained asexual from its inception without interludes of sex.
This latter attribute is the most difficult to establish and several taxa once assumed to be asexual have been shown to engage in cryptic sex. This includes fungi classified as arbuscular mycorrhizae (Glomeromycota), originally believed to be the eldest asexuals at ca. 400 million years (Kuhn et al. 2001; Croll and Sanders 2009; however, see Taylor et al. 2015). Males, hermaphrodites, and meiosis are unknown in a large metazoan taxon (Class Bdelloidea of the Phylum Rotifera), believed to be at least 35 million years old (Welch and Meselsen 2000; Flot et al. 2013). Examples of other kinds of evidence used to infer not only asexuality but in some instances ancient asexuality are several, including: (i) the independent evolution of two alleles at any given locus.
This increasing allelic divergence due to the accumulation of neutral or possibly adaptive (Pouchkina-Stantcheva et al. 2007) mutations is called the ‘Meselson effect’ (Welch and Meselson 2000; Butlin 2002); (ii) high taxonomic rank with abundance of species; (iii) phylogenetic congruence of gene genealogies; (iv) strong correlation among alleles at multiple, polymorphic loci (linkage disequilibrium) leading to the recovery of the same multilocus genotype through time and often over great distances; and (v) decay of sex- and recombination-specific genes (Tibayrenc et al. 1991; Taylor et al. 1999a,b, 2015; Normark et al. 2003). These and other tests vary in rigor, are subject to caveats, and the outcome may be subject to interpretations other than a conclusion of asexuality; for insightful discussion, see Taylor et al. (1999b) (Fig. 2.8).
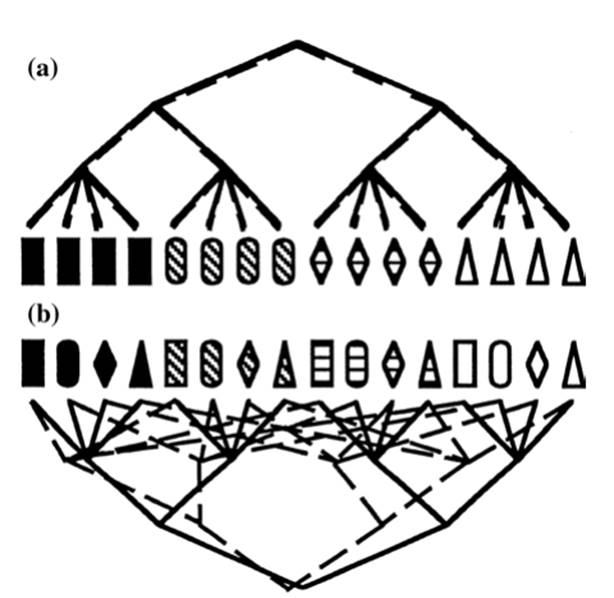
Fig. 2.8. A simplified example of strictly clonal versus recombination reproductive modes and resulting phylogenies based on two hypothetical characters, shape and pigmentation (shape = rectangles, triangles, etc. symbols representing the four structures shown; pigmentation = degree of blackness or whiteness within the structures). In the clonal organism (a), recombination is absent; the entire genome remains intact through generations and occurs in few combinations as represented by the sub-clones showing constant association between degree of pigmentation and specific shape. In the recombining organism (b), pigmentation and shape are found in all possible combinations. Note, however, that genetic regions may be constantly associated for reasons other than clonality; likewise, lack of association of loci may occur for reasons other than recombination. From Taylor et al. (1999b); reproduced from Clinical Microbiology Reviews by permission of the American Society of Microbiology, ©1999
At least in contemporary time and quite likely also in geological time, many lineages have effectively integrated alternating rounds of sexual and asexual reproduction into their life cycles. At the level of the individual or species, whether sexual or asexual reproduction dominates the cycle, or indeed occurs exclusively, depends on factors such as the local environment and whether a compatible mating type also occurs. In an evolutionary context, this is perhaps particularly true of pathogens or parasites (Price 1980) and fungal pathogens especially (Andrews 1984; Heitman 2006). Sexual reproduction is often associated with adverse environments and its occurrence in some facultatively sexual fungi is probably a specific instance of the broader phenomenon known as ‘condition-dependent’ or ‘fitness-associated’ sex reported in various taxa (see Hadany and Otto 2009). Tsai et al. (2008) quantified the occurrence of sexual and asexual rounds in populations of the wild yeast Saccharomyces paradoxus and found that a sexual cycle occurs about once in every 1,000 asexual generations.
Generally speaking, trade-offs are evident in a sexual/asexual cycle, where production of the sexual spore form typically takes significantly longer (weeks vs. days) but may be more resistant to desiccation, whereas the asexual form is produced in much greater abundance. In the case of plant pathogens, timing of the life cycle phases is typically exquisitely linked to susceptible phenological stages of the host. Repeated rounds of asexual reproduction allow the pathogen to ‘track’ the host in time and space; regular episodes of sex (for pathogens of plants, these typically occur during the over-wintering or dormant phase) allow for generation of novel genotypes to respond to selection pressure of the evolving host. Taylor et al. (1999a) suggest that fungi also may be thought of as mosaics of recombining and clonal populations: the sexual populations to be found on wild, heterogeneous hosts where the recombined fungal genotypes are generated and then move to genetically uniform agricultural hosts, where asexual populations cycle.
Heitman (2006, 2010) and Taylor et al. (2015) review several interesting cases, including that of the opportunistic human pathogen Cryptococcus neoformans. Originally believed to be exclusively asexual, this yeast is now commonly referred to by its sexual state (teleomorph), Filobasidiella neoformans (Webster and Weber 2007, pp. 660-665). It inhabits trees, soil, and pigeon guano worldwide. However, opposite-sex mating between mating types a and a has been found recently in restricted habitats in India and sub-Saharan Africa, but likely occurs much more widely. Moreover, same- sex mating (self-fertility) also occurs and generates genetic recombinants that pose a hazard for the immuno-compromised AIDS population in this area of the world.
Date added: 2025-06-15; views: 268;
