Sex and Adaptive Evolution in Prokaryotes
Not only do bacteria engage in sex, but if one means by the term that genes from different sources are recombined in a single entity, then “bacteria are particularly sexy organisms,” to use the words of Levin (1988), and subsequently, with colleagues (Johnsen et al. 2009) that “... Bacteria may not have sex often, but when they do it can be really good, at least evolutionarily speaking”! A distinctive attribute of bacterial sexuality is that sex is not formally linked to reproduction, whereas in eukaryotes there is a linkage. Prokaryotes reproduce asexually (clonally and in most species, though with notable exceptions [Angert 2005] by binary fission, see later section and Fraser et al. 2007) and the current consensus is that they undergo recombination sporadically. An extension of Levins wry comments would be that, the degree of bacterial sex apparently varies considerably among populations and species, ranging from the arguably relatively promiscuous Neisseria to the relatively asexual Pseudomonas syringae (Sarkar and Guttman 2004) or Salmonella (Maynard Smith et al. 1991, 1993; Feil and Spratt 2001; however, see Tibayrenc and Ayala 2015). Overall, recombination now appears to be the norm rather than the exception, at least among pathogens (Maynard Smith et al. 2000; Touchon et al. 2009; Bobay et al. 2015).
Unlike recombination in macro organisms that characteristically involves two complete genomes, recombination in prokaryotes is asymmetrical, typically involving a relatively large and a small donation, respectively, from the two partners. This entails replacement of small nucleotide regions in the recipient cell by corresponding regions moving almost always in unidirectional fashion from the donor bacterium. Furthermore, prokaryotic sex involves, in addition to chromosomal genes, various accessory genetic elements differing in their degree of mobility and autonomy, ranging from phages, plasmids, and transposons at the high end to genomic islands and integrons at the other (Levin and Bergstrom 2000; Touchon and Rocha 2016). These and related mobile elements have been called a “motley riff-raff of DNA and RNA fragments” (Dawkins 1982, p. 159). The ability of bacteria to routinely accept DNA from other species and even entire genes and gene clusters appears to far exceed the capability of eukaryotes in doing so.
While the three processes involved in prokaryotic sex—transformation, transduction, and conjugation—are distinct from each other and from eukaryotic sex, all produce effectively the same end result: acquisition of and usually recombination of DNA from genetically different individuals (cells). Each mechanism is quite complicated in detail and beyond the scope of this discussion. The synopsis here is intended to provide a basis for comparisons between prokaryotic and eukaryotic sex; specifics are available in general microbiology texts and advanced treatises (e.g., Levin 1988; Neidhardt et al. 1990; Bushman 2002; Madigan et al. 2015). Regardless of the process, the entering DNA may either (i) become degraded by restriction enzymes; (ii) replicate by itself (if it has its own origin of replication, as in the case of phage or plasmids) or; (iii) recombine with the recipient’s chromosome by homologous recombination.
Occasionally, it may recombine as mediated by phage integrases or mobile element transposases, or by various ‘illegitimate’ or nonhomologous means such as by double-strand break repair (Ochman et al. 2000). These latter mechanisms pertain particularly to incorporation of sequences by horizontal gene transfer. In practice, because of the limitations of detection methods, it may not be known which of the three processes is responsible for recombination in a given situation. The presence of synteny (gene blocks similarly arranged in the species compared) within and surrounding the genome break points, as well as absence of viral- (phage) related sequences, is usually sufficient to eliminate transduction. If the bacterium is naturally transformable (below), it is very difficult to separate transformation from conjugation.
In transformation, a bacterial or archaeal cell takes up naked DNA from the surrounding medium (originating usually from a lysed or decomposing cell) which is then integrated into and replicates with the recipient’s genome (Fig. 2.1). The amounts of genome transferred vary over an order of <1-100 kilobases.
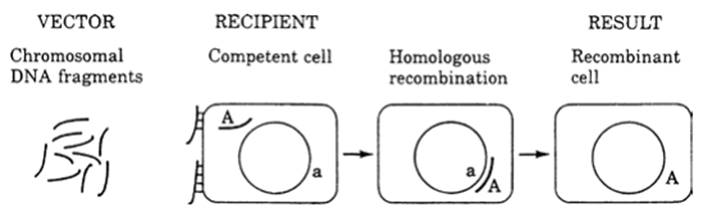
Fig. 2.1. Gene transfer in bacteria by acquisition of free DNA (transformation). Incoming chromosomal fragments from the environment (dark lines) bind to bacterial cell (rectangle); one enters the bacterium and is incorporated into the genome (light circle) by homologous recombination. From Levin (1988); reproduced by permission of Sinauer Associates, Inc., Sunderland, MA ©1988
The mechanism hinges on several conditions, among them development of a transient ability (competent state) by the recipient to be transformed. Competency is a complex trait and the selection pressures for the genes involved are unclear (Levin and Cornejo 2009; Johnston et al. 2014) though recognition and uptake of foreign DNA are highly evolved processes. Transformation occurs naturally and has been documented in many genera, including Streptococcus, Staphylococcus, Hemophilus, Neisseria, and Pseudomonas (Levin 1988; Madigan et al. 2015). Even within such genera only certain species and strains are transformable and under specific conditions. For instance, in B. subtilis, competency occurs in a small percentage of cells as they enter stationary phase of growth and is a stochastic phenomenon. It is one of several examples of bistability (see Chap. 7 and Dubnau and Losick 2006).
In nature, transformation would appear to be potentially most significant in habitats where DNA in dead and lysed cells can be protected from digestion (e.g., by adsorption to a matrix such as clay particles) and where cells occur densely, as in biofilms (see, e.g., Hall-Stoodley et al. 2004; Hiller et al. 2010). Some authors have speculated that transformation may not even be primarily a sexual process but rather has evolved to provide the cell with nutrients (Redfield 2001), though this opinion has been challenged (Johnston et al. 2014).
Others postulate that its most important function is to acquire genes from without as a source of variation (Levin and Bergstrom 2000) and specifically to restore (‘reload’) genes lost or degraded in a local population though still present in the overall population at large or metapopulation (Szollosi et al. 2006). Under simulation conditions, Redfield (1988) showed that even when the acquired DNA is from dead cells, transformation can reduce mutational load and transformed populations had a higher mean fitness than asexual populations. It is also noteworthy that competent, nongrowing cells may have a transient selective advantage over noncompetent, dividing cells under episodic conditions that kill growing cells (Johnsen et al. 2009). The striking evolutionary dexterity of the human pathogen Streptococcus pneumoniae has been attributed to gene transfer among strains by transformation (Hiller et al. 2010; Croucher et al. 2011).
This is not only interesting from the standpoint of basic microbial ecology but has important practical implications for understanding the pathogenesis and epidemiology of diseases caused by S. pneumoniae. The genomic analysis shows that this lineage evidently acquired both drug resistance and evolved adaptations (antigen switches) to counter vaccine pressure multiple times (Croucher et al. 2011). This bacterium is a natural resident commensal of the human nasopharynx and also exists as a potentially invasive pathogen. It habitually causes ear infections of children as well as frequently fatal infections such as meningitis, bacteremia, and pneumonia.
Transduction occurs when bacterial DNA is packaged within the protein capsid of a bacteriophage particle and injected into a recipient cell during the viral infection process (Levin 1988; Madigan et al. 2015; Salmond and Fineran 2015). In generalized transduction, chromosomal genes from the donor bacterium are transferred when a small proportion of progeny phage carry some random portions of bacterial DNA instead of phage DNA. The donor’s genes must recombine with homologous sequences in the recipient’s chromosome, otherwise they will be lost. In specialized transduction, temperate phage move specific, adjacent bacterial genes when the occasional phage genome excises imprecisely from its latent or prophage state in the bacterial chromosome at the onset of the lytic cycle (Fig. 2.2). Transduction has been shown to occur in numerous genera of soil and aquatic bacteria under nonsterile experimental conditions, though not all bacteria are transducible and not all phages can transduce. Nevertheless, since many phage can infect diverse bacterial species, DNA can be moved across significant evolutionary distances. Transduction is a fortuitous process, essentially resulting from mistakes in phage growth.
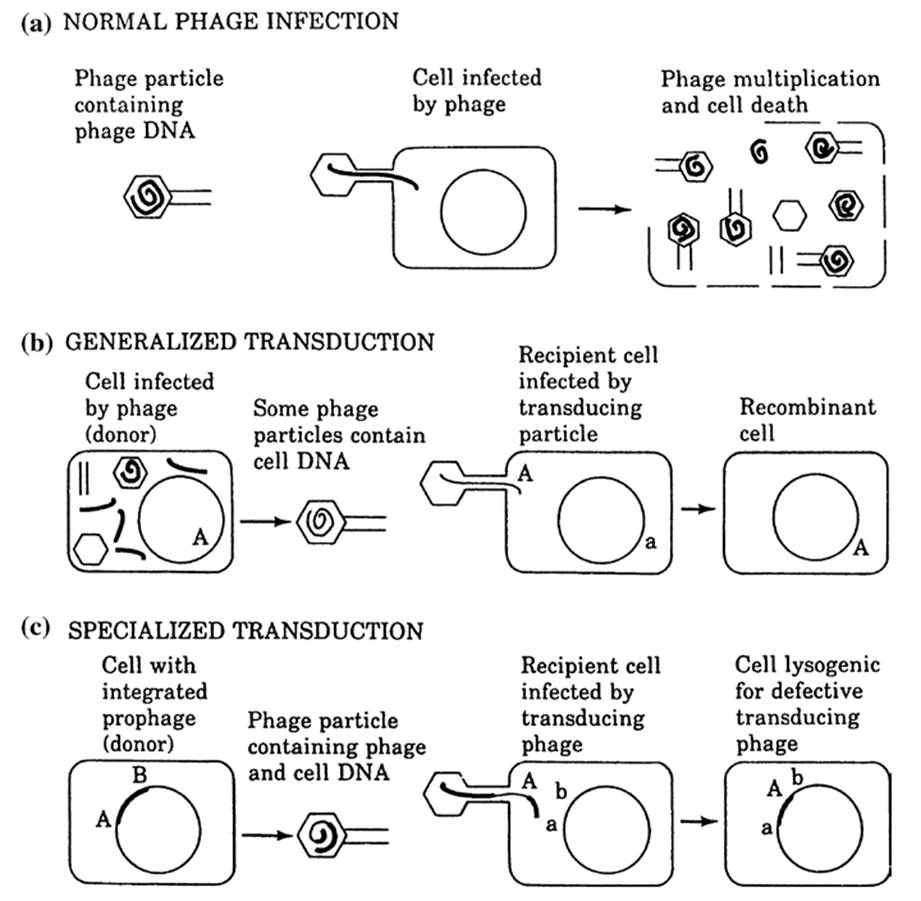
Fig. 2.2. Three possible results of infection of bacteria by bacteriophage (transduction). Heavy lines = phage genome; light lines = host genome. a In normal phage infection by lytic phages, replication of the phage leads to packaging of phage DNA in phage particles. b In generalized transduction, a few of the progeny phage contain random portions of bacterial DNA instead of phage DNA. These progeny phage then transfer the host DNA into new cells where it replaces the recipient’s genes. c In specialized transduction, part of the phage genome is replaced by adjacent host genes when the phage excises from the bacterial chromosome; these genes are inserted by site-specific mechanisms when the virus enters the genome of its new host bacterium. From Levin (1988); reproduced by permission of Sinauer Associates, Inc., Sunderland, MA ©1988
Conjugation involves cell-to-cell contact and is controlled by genes carried on certain so- called ‘conjugative plasmids’. The result is transmission from donor to recipient of plasmid (extrachromosomal) DNA alone or, occasionally, both plasmid and various lengths of chromosomal DNA (Fig. 2.3). Furthermore, there is a diverse group of mobile genetic elements maintained largely as part of the chromosome that can also be excised and transferred to another cell during conjugation but which, unlike plasmids, cannot replicate autonomously.
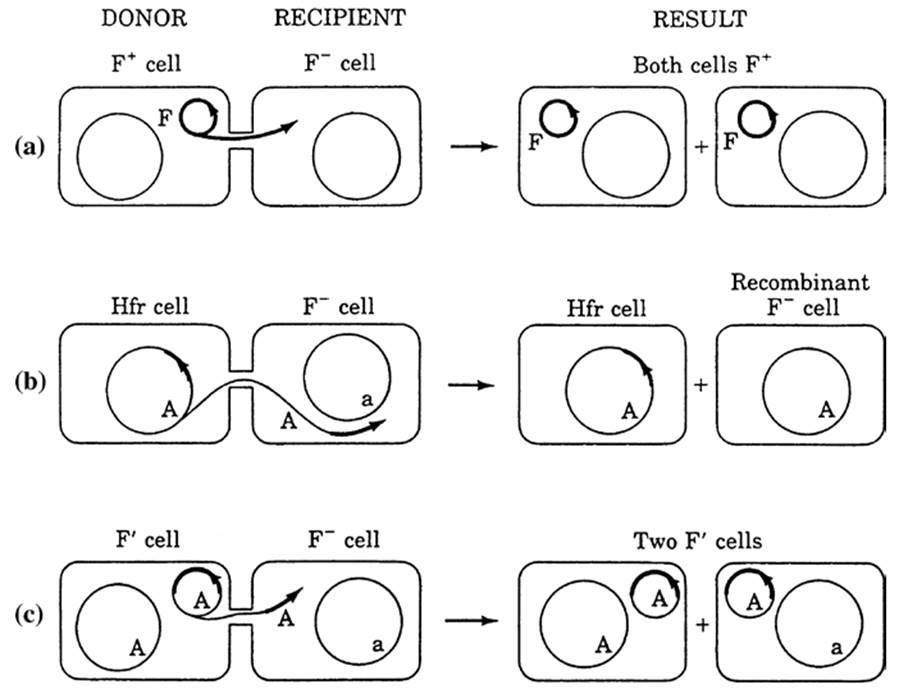
Fig. 2.3. Three mechanisms for gene transfer in bacteria as mediated by F plasmids (conjugation). Heavy lines = plasmid genome; light circle = host chromosome. Donor and recipient cells are shown united by the pilus bridge and the results of the conjugation events are indicated. a F plasmid conjugation: Only a copy of the F plasmid is transferred but not incorporated into the bacterial chromosome.
b Hfr-mediated transfer: The F plasmid is part of the donor host chromosome (in Hfr cells, see text). During conjugation, a copy of some of the donor chromosome is transferred and may replace part of the recipient’s chromosome. c F′ (prime) transfer: In rare cases the F plasmid may excise from the chromosome carrying some of the host chromosome along at which point it becomes known as an F′ plasmid. When conjugation subsequently occurs, it can transmit the host genes it acquired as well as itself. In this case, the plasmid may not integrate into the host chromosome and the recipient becomes diploid for the newly acquired chromosomal genes. From Levin (1988); reproduced by permission of Sinauer Associates, Inc., Sunderland, MA ©1988
These are collectively referred to as ‘integrative and conjugative elements’ (Wozniak and Waldor 2010). The best known of the conjugative plasmids is the F (for fertility) plasmid of E. coli and closely related enteric bacteria. One of the proteins specified by a cluster of F genes is for the sex pilus, a temporary projection that joins the F+ cells and F- cells and through which plasmid DNA moves. The result of mating is two F+ cells. In rare F+ cells (known as Hfr = high frequency of recombination), where the F particle is integrated into the bacterial chromosome, chromosomal genes also are transferred. The F particle can also mobilize a class of nonconjugal plasmids when both occur in the same donor cell. However, to put these events in perspective, Hfr formation, even under laboratory conditions, is a comparatively rare event. Such Hfr’s, once formed, are relatively unstable because the F factor excises at a high frequency. Therefore, the contribution of this type of genetic exchange to variation of bacterial populations in nature is unclear.
While the Hfr’s may be a relatively rare phenomenon, transfer of the F plasmid is not, the process occurring rapidly and efficiently and rapidly spreading the plasmid infectiously within a population (see Sidebar). In another rare phenomenon, recombination may occur between a site on the F+ plasmid and a site on the host chromosome resulting in what becomes known as a F' (F prime) plasmid containing host genes. When an F' mates with an F- cell usually the entire F plasmid is transferred and the result is a partial diploid, i.e., for the genes carried on the F plasmid as well as on the recipient’s chromosome. Plasmids can also be transferred by transduction and transformation as well as by conjugation.
As alluded to earlier, plasmids are ubiquitous among bacteria (Funnell and Phillips 2004; Touchon and Rocha 2016) and constitute the most common form of semi-autonomous replicating pieces of DNA (so-called replicons). Bacterial cells may carry more than 20 of these elements. Some may be very large (on the order of Mb DNA) and differ little from secondary chromosomes (see footnote under summary below and Touchon and Rocha 2016). In the older literature those plasmids that could integrate into the chromosome were called episomes. Bacteria lacking them generally multiply normally under laboratory conditions; hence, plasmid DNA seemingly does not encode essential functions and may be best regarded as a desirable albeit expendable source of accessory traits. However, ‘essential’ should be qualified as what appears to be nonessential under laboratory conditions, where such assessments are made, may well be essential in nature. Moreover, exchange of key genes among replicons and the chromosome may well lead to acquisition of essential genes by such elements and thereby persistence in the bacterial lineage.
To the extent to which plasmids contribute to bacterial competitiveness, they also benefit themselves indirectly by enhancing their representation in the population of bacterial carriers. Plasmid DNA contributes to the genetic plasticity of the carrier and confers many characteristics of adaptive value in diverse environments. The best known of these is antibiotic or heavy metal resistance (R factor plasmids; see Sidebar); others include the ability to induce tumors in plants (Agrobacterium tumefaciens), nitrogen-fixing capability (Rhizobium spp.), increased virulence (Yersinia enterocolitica), and antibiotic synthesis (Streptomyces spp.).
Transfer of plasmids has been observed between bacterial strains, species, and, in some instances, even between unrelated genera (Funnell and Phillips 2004). Remarkably, in the case of plant tumors (the crown gall disease, above), the mobilization function of a bacterial plasmid promotes its transfer to plant hosts: Not only can this plasmid move among bacteria, but plants have access to the gene pool of at least some bacteria (Buchanan-Wollaston et al. 1987; McCullen and Binns 2006). While plasmids may be the key means by which bacterial genes are transferred in nature, it is not yet clear that conjugation is the mechanism involved, although this is generally inferred to be the case.
The evidence has been reviewed (Levin 1988; Touchon and Rocha 2016) for plasmid- and phage-mediated bacterial sex being simply coincidental to the infectious (parasitic) transfer of the elements involved and the availability of recombination repair systems in the bacterial hosts. The population biology of the highly mobile elements such as plasmids prompts many interesting questions as to the selection pressures favoring their survival and genetic options for the host bacteria. For example, since plasmids impose at least a minimal fitness cost on the host, it should be advantageous from the plasmid’s perspective to maintain genes that are at least occasionally beneficial to the bacterium. Likewise, there are some situations where it may be more or less advantageous for the bacterium to have its genes either integrated into the chromosome or on a mobile (infectiously transmitted) vehicle (Bergstrom et al. 2000).
In an historical context it worth a note in passing that key aspects of bacterial sexuality were discovered by Francois Jacob at the Pasteur Institute in the 1950s and 1960s, who, with his colleague, Elie Wollman, devised a simple but elegant technique for mapping genes in a linear sequence. Using a Waring blender to interrupt mating bacterial cells at sequential times during the process, they found that different genes were transferred in a specific order and could be mapped as to position. This also revealed the circular nature of the bacterial chromosome and that episomes can be added to or subtracted from the bacterial chromosome (see especially Chap. IX in Jacob and Wollman 1961). For this and related brilliant work on gene regulation and his ingenious deductions, Jacob shared the 1965 Nobel Prize in Physiology or Medicine with his colleagues Jacques Monod and Andre Lwoff. (Jacob’s Nobel lecture is well worth reading, as is his eloquent autobiography, The Statue Within.)
Bacterial sexual reproduction, as reviewed above, serves to propagate the variation in existing genes by so-called vertical transmission (in the course of cell division) and incorporation through homologous recombination of such novelties as mutational changes, gene rearrangements, and related intra-genomic alterations (Gogarten et al. 2002). An important extension of the sexual process called lateral or horizontal gene transfer (HGT) (Ochman et al. 2000), alluded to in various earlier contexts, involves the integration within a recipient cell of entire genes or gene clusters that are fundamentally new (exogenous) to the genome. Unlike vertical transmission, in HGT the donor and recipient may be distantly related prokaryotic species or genera, at the extreme even in different domains (for transfers from prokaryotes to eukaryotes or among eukaryotes see Dunning Hotopp et al. 2007; Keeling and Palmer 2008; Andersson 2005, 2009; Gilbert et al. 2010). The sequences transferred tend to retain characteristics of the donor and so can be distinguished from ancestral DNA (for a discussion of how such inferences are made, see Ochman et al. 2000). Traits introduced by HGT frequently are complex phenotypic features including antibiotic resistance, virulence, and metabolic properties.
HGT has profound implications for organism taxonomy and phylogeny as well as evolutionary ecology. With respect to the former, prokaryotic species boundaries (Rossello-Mora and Amann 2001; Rosen et al. 2015) tend to become blurred (Ochman et al. 2005; Shapiro et al. 2012). Thus, bacterial species might better be thought of less rigidly as “distinctive arrays of varying but co-adapted gene complexes which are periodically reshuffled” (Duncan et al. 1989, p. 1586). Perhaps more significantly, the evolutionary history of a gene does not necessarily equate with that of the organism. This is most dramatically seen in the contentious debates surrounding attempts to root the universal tree of life (Brown 2003; Chaps. 4 and 27 in Barton et al. 2007; Chaps. 1 and 4 of this text). A quantitative measure of the prevalence of lateral transfer is provided by Lawrence and Ochman (1997), who examined a sequenced region of about 30% of the E. coli chromosome.
Based on atypical base composition and codon usage patterns, they estimated that at least 17% of the protein-coding sequences resulted from HGT since the divergence of Escherichia from Salmonella (or more than 600 kb of transferred DNA accumulated at the rate of about 31 kb per million years; smaller estimates have been made in other systems). This was viewed as being quantitatively similar to the amount of variation introduced through mutation. However, qualitatively, as alluded to above, the impact of the two processes is very different, with HGT potentially providing novel functions to the recipient, i.e., a significantly changed phenotype, immediately, and ultimately being a process that enhances genome dynamics and diversifies lineages (Nowell et al. 2014). Indeed, as an extreme example, it has been proposed recently that adaptation of Archaea, which were originally hyperthermophiles, to a mesophilic lifestyle is attributable to HGT from the Bacteria (Lopez-Garcia et al. 2015).
The relationships among several enteric bacteria are shown in Fig. 2.5. Frequently, the key traits defining those relationships have resulted from genes transferred by HGT. For example, E. coli acquired the lactose operon, hence the ability to utilize lactose and thereby colonize the intestinal tract as a commensal, while genes conferring pathogenicity islands in Salmonella and Shigella also are conveyed horizontally (Ochman et al. 2000; Gogarten et al. 2002). So lineages can separate as a result of HGT and entirely new niches are created as opposed to being simply refined. The implications can be visualized in terms of Wright’s classical model (1932) portraying a population at successive peaks on an adaptive landscape. Having climbed a peak, an evolving population would tend to stay there because to descend would mean to decline in fitness. As Gogarten et al. (2002) point out, peaks may never be explored if they can be reached only by changing one gene at a time (i.e., by mutational processes). HGT can overcome this constraint by introducing multiple changes simultaneously.
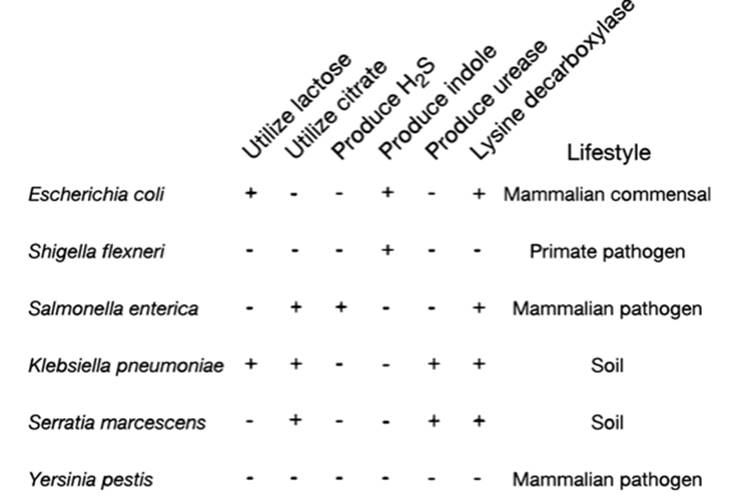
Fig. 2.5. Representative phenotypic properties of, and evolutionary relationships among, certain enteric bacteria based on nucleotide sequence data (+ = presence; - = absence of the trait). Many such species- specific traits are conferred to bacteria, not by conventional mutation or the reshuffling of existing genetic information, but by the acquisition of sequences through horizontal gene transfer (HGT) from distant relatives. Note in particular the differences between E. coli (mammalian commensal) and its sister species S. enterica (mammalian pathogen). These traits and others are inferred to have arisen from HGT. From Ochman et al. (2000); reproduced from Nature by permission of Macmillan Publishers, Ltd. ©2000
To summarize, sexual reproduction and adaptive evolution in prokaryotes is loosely analogous to that in the sexual eukaryotes with some important qualifications. Unlike most eukaryotes, the prokaryotes reproduce for the most part asexually (clonally) as discussed further under 2.4 The Asexual Lifestyle, later. Clones tend to progressively diverge genetically by the sequential accumulation of mutations. Of course they also diverge as a result of recombination or acquisition of foreign DNA from distant phylogenetic sources by horizontal transfer. Whether at the point of acquiring foreign DNA from close or distant sources a cell is still considered to be a clonal member is a matter of definition; see later section and Tibayrenc and Ayala (2012). Bacteria are considered to be haploid because generally there is only one copy usually of one chromosome present. Any genetic change is thus immediately expressed and exposed to natural selection. Genes are exchanged over a substantially wider phylogenetic range and routinely in the prokaryotes unlike the case among eukaryotes.
This provides both for the variation in existing genes typical of eukaryotes (by homologous recombination) but, more significantly, the wholesale introduction of unique traits by HGT and nonhomolo- gous recombination allowing major changes in organism habitat or niche. Moreover, the sexual process is mediated by a diverse array of semi-autonomous accessory genetic elements that do not appear to play a significant role in eukaryotes.
Selection pressure on microorganisms, and prokaryotes in particular, typically favors a small genome and rapidity of reproduction. The evolution of prokaryotic chromosome structure and evolution of genome organization are separate but closely related and interdependent processes (Touchon and Rocha 2016).
The genome is a streamlined, plastic, dynamic one where there is tension between high plasticity at one extreme and high organization at the other. One gets the impression of a busy railroad station, a scene of frenetic activity with the continuous comings and goings trains and throngs of passengers who intermingle but in an organized manner. Though the population size of prokaryotes typically is vast, dwarfing that of eukaryotes, the effective genetic size can be lower because of bottlenecks or the recurrent selective sweeps of mutants through a population (see comments on asexuality, later, and Reeves 1992; Maynard Smith et al. 2000; Levin and Bergstrom 2000).
A Case Study: Transferable Drug Resistance in Bacteria
A central message of this chapter is that although all living things have generally analogous means of generating and transmitting genetic variation, the potential evolutionary rates of microorganisms are much higher than those of macroorganisms. This is a function of their short generation times, hence large population sizes (Chap. 4), widespread dissemination and mixing, efficient means of genetic exchange, numerous accessory genetic elements, and the relatively broader phylogenetic range over which gene exchange occurs.
A classic example of natural selection in action (evolution in changing environments) is the phenomenon of antibiotic resistance in bacteria. Such resistance is either encoded by chromosomal genes or plasmid-borne (genes typically on one class of conjugative plasmids known as resistance or R factors) (Nikaido 2009). Plasmids are especially significant ecologically because resistance to several different antibiotics can be combined in a single element, which can also serve a role as an efficient vector as well as mediating genetic rearrangement (Koch 1981; Sandegren and Andersson 2009). In the presence of an antibiotic, the plasmids may increase in size due to gene duplication and/or in plasmid copy number per cell; i.e., the antibiotic resistance genes can be amplified when necessary and deamplified when not needed (Sandegren and Andersson 2009). Early stages in the evolution of resistance of E. coli to ciprofloxacin involve extensive cytological changes, including the production of multi-chromosome-containing filaments (Bos et al. 2015).
In the presence of an antibiotic(s), the drug-resistant phenotype obviously has a selective advantage. In absence of the drug, there are typically fitness costs associated with resistance manifested as a decline in growth rate or virulence, but bacteria often respond by compensatory mutations at other loci or amplification of the affected gene (Gagneux et al. 2006; Andersson and Hughes 2010). Amelioration of fitness costs in the absence of the drug commonly result in a bacterial population that is fitter in drug-free culture than the uncompensated resistant population, but less fit that the original wild-type. Levin et al. (2000) speculate that the common ascent of intermediate-fitness compensated mutants, rather than high-fitness revertants, may be attributable to higher rates of compensatory mutation relative to reversion and to bottlenecks in culture associated with serial passage. From a microbial ecology standpoint, as well as with respect to the strategy of antibiotic administration, the fact that reversion to sensitivity is difficult has important implications (e.g., Tanaka and Valckenborgh 2011).
Resistance to many if not most antibiotics is known, and frequently the genes responsible are carried within transposons. Conjugative plasmids typically replicate during transfer; hence, acquisition by the recipient is not at the expense of loss from the donor. The resistance genes spread so quickly (often exponentially) that the phenomenon has been called infectious drug resistance. For instance, following the introduction of antibiotic therapy with streptomycin, chloramphenicol, and tetracycline from 1950 to 1965 in Japan, the proportion of drug-resistant Shigella (the bacterium that causes bacillary dysentery) increased from about 1-80% of the isolates (Mitsuhashi 1971).
The history of the use of various antibiotics to control Staphylococcus aureus is analogous. Waves of resistance dated from the introduction of penicillin in the 1940s, through other й-lactam antibiotics, in particular methicillin. S. aureus can cause rapidly progressing, potentially fatal skin and soft-tissue infections. This situation has culminated with worldwide epidemics of methicillin-resistant Staphylococcus aureus (MRSA) clones that spread rapidly among healthy individuals, as opposed to earlier outbreaks associated mainly with hospitals and similar settings (Chambers and DeLeo 2009) (Fig. 2.4).
In a parallel situation, the genomic plasticity and hence evolutionary versatility of Streptococcus pneumoniae to multiple antibiotic and vaccine pressure has been thoroughly documented. This was approached by large-scale genomic analysis of a single ancestral strain (clone) by sequencing 240 isolates of the lineage PMEN1 from 22 countries (Croucher et al. 2011). Their approach allowed relatively easy determination of the number and size of base replacements, and thereby in separating variation due to recombinations from point mutations. The clone has diversified rapidly since its estimated origin in 1970. Base substitutions occurred about once every 15 weeks; though recombination events occurred at about one-tenth that rate, they introduced an average of 72 single nucleotide- polymorphisms each, with 5% of the replacements involving more than 30 kb of genome. Overall, 74% of the genome length had received a recombination event in at least one isolate. On average, some 74,000 bp of sequence were affected by recombination in each strain. A related intensive study of recombination events among four strains in the nasopharynx in a single patient repeatedly sampled over a much shorter period (7 months) confirms the rapid evolutionary potential of S. pneumoniae (Hiller et al. 2010).
At least 156 kb corresponding to about 7.8% of the genome was exchanged, probably by transformation, during the multiple recombination events. The authors suggest that this case supports the 'distributed genome hypothesis,' which proposes that: (i) bacterial species consist of multiple, co-occurring, complementary strains among which the fluid 'pangenome' or species-level genome is distributed (Ehrlich et al. 2010); and (ii) pathogen genetic diversity accomplished by concurrent infection with multiple strains (so-called polyclonal infection) can overwhelm a host's immune response (Hiller et al. 2010).
The activity of diverse mobile genetic elements in the origin and spread of resistance genes provides an interesting case study in the plasticity of bacterial evolutionary processes.
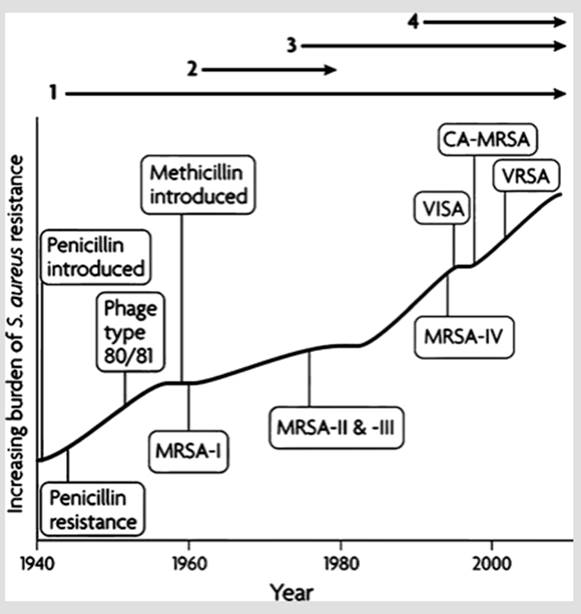
Fig. 2.4. Four major epidemic waves of antibiotic resistance in Staphylococcus aureus with the respective eras shown above the graph. Wave 1 began in the mid-1940s shortly after the introduction of penicillin, driven by strains producing a plasmid-encoded penicillinase. The second wave following within 2 years after the introduction of methicillin in 1959 was caused by a strain designated here as MRSA-I (for methicillin-resistant S. aureus) that carried a gene encoding a low-affinity penicillin-binding protein that confers resistance to the entire class of β-lactam antibiotics.
Wave 3 began in the mid-late 1970s with clones derived from earlier infections as well as new lineages; the use of vancomycin to treat MRSA infections led to vancomycin-intermediate S. aureus (VISA). Wave 4 began in the mid-late 1990s and marked the emergence of MRSA strains with distinct attributes in the community at large (CA-MRSA) as opposed to clinical settings. Strains tolerating even higher doses of vancomycin are called vancomycin-resistant S. aureus (VRSA). From Chambers and DeLeo (2009); reproduced from Nature Reviews Microbiology by permission of Macmillan Publishers Ltd. ©2009
To the role of conventional point mutation in molding the genome must now be added such dynamic processes and factors as phage transduction, transposons, plasmids, horizontal gene transfer, pathogenicity islands, and probably others awaiting discovery. Transfer of drug resistance is not only an important phenomenon within the context of basic microbial ecology, but obviously has profound implications in practical terms of how antibiotics can be over-prescribed in medicine. Furthermore, because drug-resistant bacteria of animal origin can cause serious diseases in humans (Wegener 2003), the routine use of antibiotics as animal feed supplements should be restricted. For additional reading on the ecology of transferable drug resistance, see Pranting and Andersson 2011; Jackson et al. 2011).
Date added: 2025-06-15; views: 204;
