Genomic Plasticity. Epigenetics and Gene Regulation
The sorts of nucleotide changes reviewed above emphasize that both protein-coding and noncoding regions are subject to dynamic evolutionary change. Cells can read multiple messages from the same DNA sequence and these do not just pertain to protein structure (Shapiro 1999, 2002). Mutations are not limited to nucleotide substitution but can be genome-wide rearrangements involving potentially large blocks of nucleotides. As Shapiro states (2002, p. 9) “... living cells can rearrange their genomes in any way that is compatible with the rules of DNA biochemistry.” Such rearrangements allow rapid genotypic and phenotypic changes by organisms, as in the response of microorganisms to antibiotic selection pressure discussed later, or by the vertebrate immune system to novel antigens. Physical changes in the genome accomplished by the rearrangements are amplified by DNA interactions with cellular complexes that do not alter the sequences (Table 2.2 and Shapiro 2002; see Epigenetics below). The bacterial genome in particular is highly plastic, with multiple mobile components of the genome interacting, being transferred, gained and lost in a dynamic equilibrium (Touchon and Rocha 2016; developed in Sect. 2.3).
Epigenetics and Gene Regulation.Epigenetic controls include nongenetic, enzyme-mediated chemical modifications of DNA structure (methylation of DNA residues after replication) and changes to the associated protein (mostly histones) (Feng et al. 2010; Griffiths et al. 2015). Both processes affect transcription and thereby gene activity. Some alterations to histone as well as DNA methylation marks can be inherited stably and such instances are referred to as ‘epigenetic inheritance’ (although terminology varies; see Eichten et al. 2014). Epigenetic changes such as erasure of DNA methylation or ‘reprogramming’ can also occur in both plants and animals where they play an important role in development (Feng et al. 2010). Classic cases of epigenetic inheritance include gender-specific gene silencing even though both the maternal and paternal copies are functional (‘genomic imprinting’), and even the silencing of an entire chromosome (random inactivation of one of the two copies of X chromosomes in female mammals).
It is perhaps in the realm of gene regulation more so than any other that Shapiro’s (2002) computational metaphor of the genome, noted above, is most apt. The inert DNA storage medium (hard drive) interacts with cell complexes to format the information in a readable, transmissible manner. Though complex in detail, regulation of the message takes many forms and is exerted at numerous points broadly directed at the transcription and post-transcription levels (Table 2.4). In bacteria, one method of control involves proteins that inhibit or activate transcription of specific genes (see discussion of the lac operon in Chap. 3). In eukaryotes, transcription is controlled in part by multiple ds-regulatory sequences, so-named because they typically are located on the same DNA molecule as the gene affected. In plants, chromatin modifications and other forms of genetic regulation influence development and reaction to environmental stimuli (Feng et al. 2010; Eichten et al. 2014).
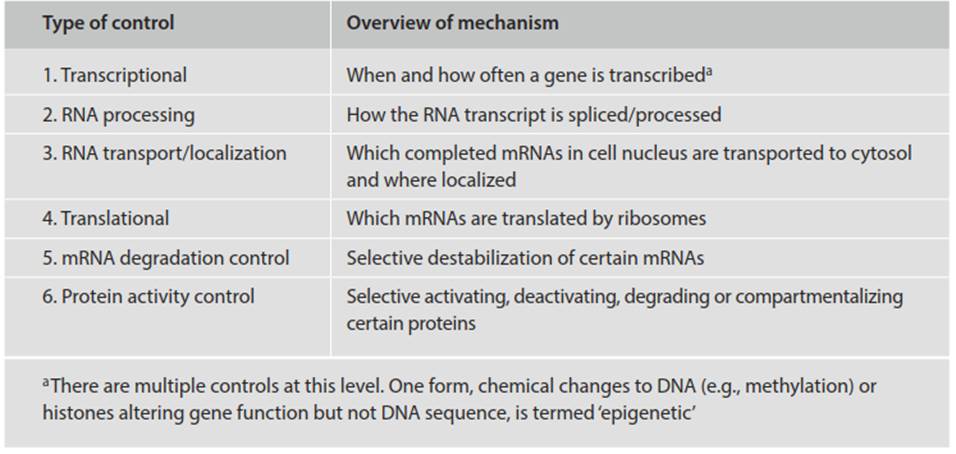
Table 2.4. Six points at which eukaryotic gene expression can be regulated (Alberts et al. 2015). Bacteria generally employ similar mechanisms
In animals, there may be 5-10 times as many such regulatory modules as there are genes (Davidson 2006). Sequence-specific DNA-binding proteins (transcription regulators), which are themselves variably active by time in the life cycle and place in the organism, read this information and determine the time and location of genes to be transcribed (Davidson 2006; Tuch et al. 2008; Gilbert and Epel 2009).
It appears from the model organisms studied to date that most if not all the eukaryote genome is transcribed and among the products are several classes of small, noncoding RNAs (ncRNAs) (Amaral et al. 2008). Two such classes of ncRNAs are termed microRNAs (miRNAs) and small interfering RNAs (siRNAs) (Amaral et al. 2008; Ghildiyal and Zamore 2009). They appear to be primarily regulatory, achieving their effect by interacting with transcription factors, RNA polymerase, or directly with DNA (Amaral et al. 2008). For example, transcription can be affected in various ways, including through various modifications of chromatin structure (Slotkin and Martienssen 2007; Figueiredo et al. 2009). The miRNAs direct mRNA degradation or repress translation (Amaral et al. 2008) and may function at multiple hierarchical levels in regulatory networks (Makeyev and Maniatis 2008; Dekker 2008).
Among the siRNAs, the so-called exo- and endo-forms (Ghildiyal and Zamore 2009) are derived from dsRNA and are associated with Argonaute (Dicer) proteins that execute the regulation. This specific form of silencing, differing in details but broadly represented among eukaryotes, is known as RNAi (interference) (Ghildiyal and Zamore 2009). At least in plants, it serves also as a form of antiviral defense (Baulcombe 2004). As a regulating mechanism on gene expression, RNA interference (RNAi) is a topic of intense research on gene silencing and has practical implications, for example, in disease and pest control (Zhang et al. 2015). Morphological evolution may depend largely on changes in gene expression accomplished by mutations in regulatory networks (Prud’homme et al. 2006, 2007; Davidson 2006; Carroll 2008), though the extent to which such changes drive evolution is controversial.
The far-reaching impact that seemingly minor or innocuous changes to the genome can have is evident in the following example: There is a class of genes (proto-oncogenes) that appears to have a normal housekeeping function within the cell but which, if altered by mutation in a coding or noncoding region, can lead to malignant transformation. In the human Ha-ras gene, a single point mutation within the fourth intron can cause a tenfold increase in gene expression and transforming activity (Cohen and Levinson 1988).
This is only one example of many that show there are several ways to change gene expression without changing the message itself (see also McDonald 1990). Undoubtedly, numerous ways of altering expression are important in evolution and may explain why humans have so many genes in common with other organisms. It was pointed out insightfully by King and Wilson in 1975 that probably humans differ from chimpanzees largely because of differences in gene expression, rather than in gene structure. As the human genome continues to be studied intensively in the years since being sequenced in 2001, it has been a surprise to find that the protein-coding regions, some 3 billion bases in all, account for only a trivial amount (about 1.5%) of the total. At least some and perhaps much (still actively debated) of the vast noncoding portion—often formerly derided as ‘junk DNA—now appears to have a critical regulatory function.
Finally, that there are many gene copies in the more complex life forms provides the opportunity for alterations in the genome by changing introns, exons, or both. New gene products can be exposed to natural selection while the organism is buffered through continued function of the unaltered product encoded at another site(s). Genes that have been rendered silent can be retrieved, and other genes turned on or off, all with the phenotypic expression of a point mutation in a coding region, but accomplished simply by changing patterns of regulation. Controlling the timing or extent of gene expression has important ecological implications because this mechanism in effect increases the phenotypic plasticity of the organism as discussed in some detail in Chap. 7. Cells of more complex organisms in particular have a large repertoire of mechanisms to generate genetic variability!
Date added: 2025-06-15; views: 216;
