Sex and Adaptive Evolution in Some Simple Eukaryotes: The Fungi
For the fungi, sex followed by meiosis is generally similar to that in the higher eukaryotes. Three distinctions with broad ramifications, however, need to be emphasized: (i) Variation among taxa in rapidity of completion of the stages of sexual reproduction—plasmogamy, karyogamy, and meiosis—establishes four basic life cycles and their associated nuclear conditions, in addition to a category putatively ascribed as nominally asexual. These patterns are summarized below along with some relevant terminology. (ii) Fungi show extreme phenotypic plasticity and can exhibit alternative or supplementary genetic systems, most notably heterokaryosis and parasexuality (also reviewed briefly below).
Thus, for the fungi, recombination need not be meiotic or sexual (for details see Taylor et al. 1999a,b, 2015; Billiard et al. 2012). A given fungal species or individual may display different modes of sexuality based on seasons of the year or geographic locations (Taylor et al. 1999a). Furthermore, both sexual and vegetative fusions enable information molecules such as plasmids and mitochondrial DNA to be exchanged among fungal thalli. The septa (cross-walls) transversing the hyphae are rarely complete in areas other than where reproductive structures are borne. Apart from the obvious physiological implications pertaining to cytoplasmic streaming, solute movement, and so forth, this means that multiple, potentially genetically distinct nuclei exist within a common cytoplasm. (iii) Sex is associated often with resting spore formation and is frequently triggered by adverse environmental conditions (see Chap. 7). For these reasons and because the fungi display the genetic characteristics of a transitional group between the prokaryotes and the more complex eukaryotes, the following comments provide background informative for discussions in later chapters.
Life cycles of the fungi and associated nuclear states, mating systems, and degree of genetic variation are numerous and typically complex (Billiard et al. 2012). Simplistically, the options might be categorized as follows (Carlile et al. 2001): (i) Asexual—an artificial assemblage of species (formerly, Fungi Imperfecti or Deuteromycota) united historically by their apparent absence (based on morphology) of conventional sexuality. Existence of a sexual cycle has recently been documented or very strongly implied from genomic or population genetics data for the human pathogens Cryptococcus neoformans, Candida albicans, and Aspergillus fumigatus, all traditionally believed to be strictly clonal (Heitman 2006, 2010; Taylor et al. 2015).
Mycologists abandoned this formal classification in 2012; the current concept is that fungi exhibit both episodes of recombination and clonality in their life cycles. More is said later about this category in the later section The Asexual Lifestyle. (ii) Haploid (haploid/monokaryotic)—the life cycle is predominately haploid and the hyphal cells generally uninucleate (monokaryotic). Karyogamy follows soon after plasmogamy. Meiosis and compartmentation of the meiotic products by septa in the hyphae follow soon after that (Ascomycota), or, if delayed, the zygote remains dormant (Zygomycota, e.g., the bread mold Rhizopus stolonifer) (iii) Haploid (haploid/dikaryotic)—the cycle is similar to (ii) except that each cell of a dikaryotic mycelium “... contains paired, synchronously dividing nuclei, one of each given by the original gametic genotypes” (Anderson and Kohn 2007, p. 345; see below). Classic examples are the rust fungi such as Puccinia graminis, discussed in Chap. 6.
The dikaryotic phase may be transient (typical of the Ascomycota) or exist for much of the vegetative phase where karyogamy is delayed after plasmogamy (common in the Basidiomycota). For example, typically in the mushroom-producing (agaric) fungi, the perennial dikaryotic mycelium grows indefinitely and hidden from view as a saprobe in the soil or thatch layer. It may give rise annually to a short-lived (days or weeks) flush of mushrooms in which the life cycle stages of karyogamy followed immediately by haploidy (meiosis and sporulation) occur.
The dikaryotic, vegetative, mycelial phase of the well-known fairy ring mushrooms common in pastures may exist for several centuries, with the rings expanding progressively outwards (Dix and Webster 1995). (iv) Haploid/diploid—the cycle alternates regularly or irregularly between these two nuclear conditions as in many yeasts. (v) Diploid—this group overlaps with (iv) and is analogous to most higher organisms where the haploid phase is relatively inconspicuous and may be relegated to the gametes. It includes members of the Oomy- cota (fungus-like organisms now considered to be a monophyletic group within the kingdom Straminipila; Webster and Weber 2007).
The vegetative cells and much of the life cycle of many yeasts are diploid (Chaps. 10 and 24 in Webster and Weber 2007) or preponderantly so. For example, the remarkable morphogenetic gymnastics of certain strains of the human pathogen Candida albicans, formerly thought to be a bland and well behaved, ‘obligate diploid’, classic yeast, illustrate how dynamic are the reproduction options of the fungi (Hickman et al. 2013). It is the variations on the theme that are informative.
Most fungi belong either to category (ii) or (iii), that is, they reproduce both sexually and asexually and are haploid for most of their life cycle. The haploid, asexual phase of the cycle is generally repeated numerous times annually, typically by rounds of sporulation but in some taxa by other asexual methods such as by budding or fragmentation of the soma. The sexual phase normally occurs only once a year, and may more or less overlap the asexual state. Although a cycle comprised of haploid and to greater or lesser extent diploid structures is thus conventional in the fungi, the alternation of generations is not distinct or regimented, unlike the case in many higher organisms.
Some fungi that engage in sex are hermaphroditic in that a single thallus can function simultaneously as both ‘male’ and ‘female’; such organisms are thus self-fertile, nonoutcrossing, and are said to be homothallic. Others are self-sterile, requiring the union of two compatible thalli and are said to be heterothallic. Across the fungal world and including the related Oomycetes, the range of sexuality includes haploid selfing, diploid selfing, and outcrossing (for details see Billiard et al. 2012). Fungi have at least two mating types and in the mushrooms there are as many as several thousand (Brown and Casselton 2001).
Dikaryosis, heterokaryosis As noted in the life cycle overview above, the dominant vegetative phase characteristic of the phylum Basidiomycota is generally a dikaryotic (n + n) mycelium. This prolonged, balanced nuclear phase is unique in the living world to certain fungi. As such it warrants some discussion.
In the basidiomycetes, typically the post-meiotic, haploid sexual spore (basidiospore) germinates to produce a filament (hypha) containing genetically identical (therefore, homokar- yotic) nuclei in uninucleate or monokaryotic cellular compartments. Hyphae tend to fuse constitutively as they grow; such fusions serve several physiological purposes as well as setting the stage for nuclear transfer in sexually or vegetatively compatible colonies (Glass et al. 2004). When such anastomoses involve different but genetically very closely related individuals of sexually compatible mating type, a developmental program is triggered that results in the dikaryotic mycelium on which the fruiting bodies later develop (Anderson and Kohn 2007; Webster and Weber 2007). This process is dictated, at least in the mushrooms, by different allelic versions of multiallelic genes at two unlinked loci, A and B; Casselton and Econo- mou 1985; Brown and Casselton 2001).
The B genes encode pheromones and pheromone receptors; the A genes encode proteins involved in transcriptional regulation and synchronized division and cellular distribution of the conjugate nuclei described below. In the ‘dikar- yotization’ process, each homokaryon acts simultaneously as male and female, both donating and receiving nuclei, which divide and migrate quickly and generally more or less widely throughout the recipient mycelium under control of the B genes. However, the mitochondria typically do not migrate, so the resultant dikaryon has a consistent nuclear background but is a spatial mosaic for cytoplasmic content, including mitochondrial DNA.
The classic dikaryon appears in the form of sexually compatible nuclei representing the original gametic genotypes, physically associated and dividing synchronously for an indeterminate period as growth ensues (Fig. 2.6). Cell divisions in the extending hyphal apex typically are associated with construction of a cytological feature known as the clamp connection, which ensures that the daughter compartments receive exactly two nuclei, one of each mating type.
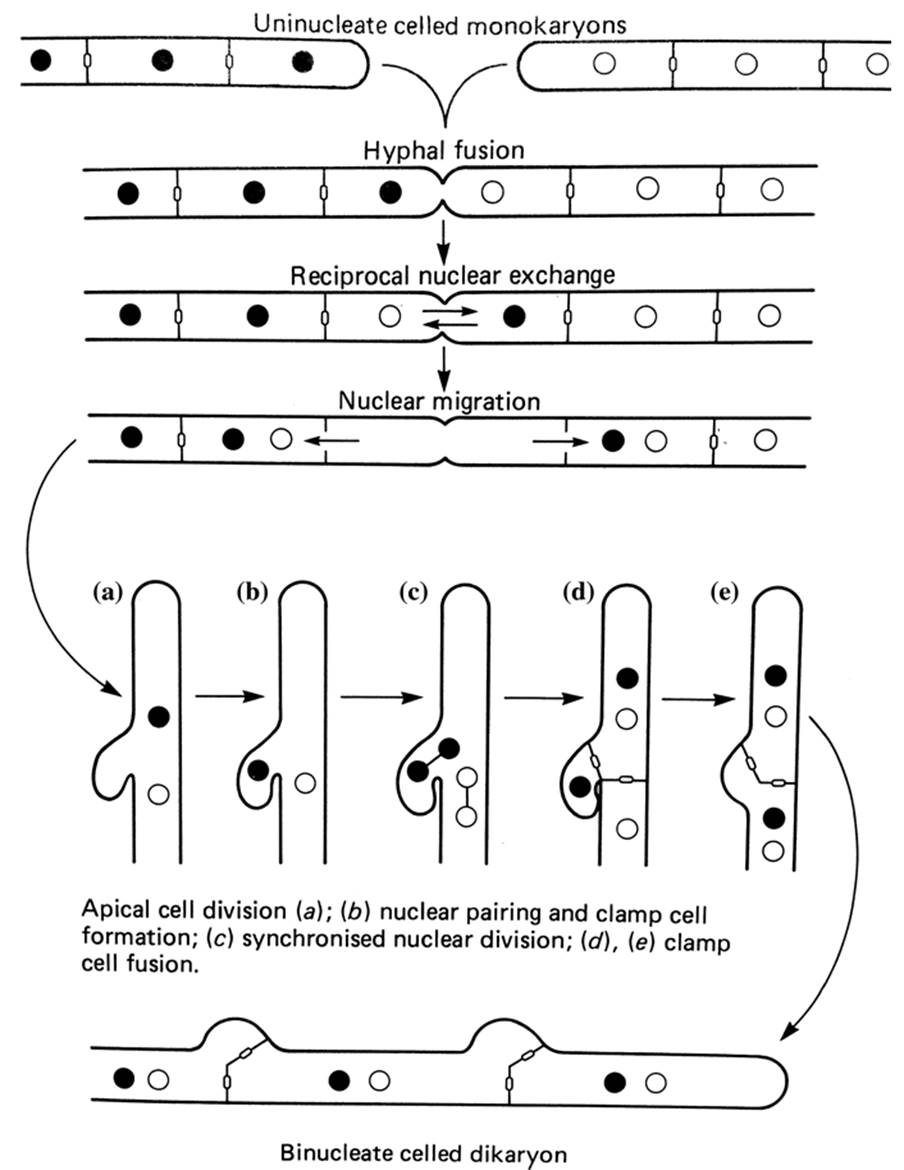
Fig. 2.6. The sequence of events leading to a fungal dikaryon from two compatible monokaryons. From Casselton and Economou (1985); reproduced by permission of Cambridge University Press, ©1985
Throughout this protracted dikaryotic phase, the conjugate nuclei remain separate but physically associated and in close molecular communication—intriguingly, the distance between them has an impact on gene expression (Anderson and Kohn 2007). The two nuclei may exchange genetic material and undergo somatic recombination (Clark and Anderson 2004; Gladfelter and Berman 2009). When dikaryotization is complete, the new genetic entity functions as a unique genetic individual and tends to rebuff through somatic incompatibility further fusions and genetic invasion by other dikaryons.
What are we to make of this fascinating quirk of nature? The expansive dikaryotic phase in the basidiomycetes may represent little more than a remnant in a general evolutionary trend to diploidy. Alternatively, this life cycle phase may be under ongoing positive selection pressure as a means for these fungi to have the phenotypic plasticity to cope with heterogeneous environments. There is some theoretical support as well as limited experimental evidence (Clark and Anderson 2004) to suggest that the latter is the case. Raper and Flexer (1970, p. 419) refer to dikaryosis as something of a biological oddity and an evolutionary cul-de-sac, although a highly successful one ...” and elsewhere (p. 417) that ... “the stable vegetative dikar- yon is not only the physiological and genetic equivalent of a diplophase, it is a far more plastic and adaptable consortium of two genomes than is the diploid.” Casselton and Economou (1985) speculate that the phenomenon of extensive bidirectional migration of nuclei allows them to be positioned for sexual fusion even where cytoplasmic incompatibility occurs (see discussion in Chap. 5). In population genetics terms, J.L. Harper (University of Wales; personal communication, 1987) has likened the phenomenon of dikaryotization to mate competition theory. Among polygamous animals there is often intense competition between males for females, which has led to the evolution of various sexual selection strategies. Analogously, in the fungi it would seem advantageous for an organism to preempt rivals by sequestering the nuclei of a compatible mate.
There are, however, even further variations on this theme of genetic versatility. They include ‘sectoring, which may occur among dikaryons restoring the monokaryotic state locally. The occasional exchange of nuclei among dikaryons has been reported, as well as the more common phenomenon of a dikaryon mating with and thereby dikaryotizing a monokaryon if it is of compatible mating type. The most noteworthy complication is that many basidiomycetes (as well as ascomycetes) form not strictly dikaryons but rather heter- okaryons with multinucleate cells (James et al. 2008). In this fluid situation the numbers of nuclei per cell are variable. The nuclei are not associated in pairs and their activities may or may not be coordinated. Thus, nuclear ratios of the parental genotypes are imbalanced (not 1:1 as in dikaryons) and nuclear competition and altered allele frequency occur in sections of a mycelium where the frequency of one nuclear type outnumbers the other. Indeed, in a fungal syncytium (multiple nuclei within a common cytoplasm) there may be thousands or even millions of nuclei of various origins, each of which is more-or-less mobile for potentially long distances, i.e., throughout the syncytium. Movement of nuclei largely by bulk cytoplasmic flow may reach several µ m/s. Each different nucleus has the potential to give rise to a new individual (Roper et al. 2011, 2013). Fusion phenomena with respect to inter-individual compatibility or repulsion, the multinucleate condition, and fungal cytology are discussed further in Chap. 5.
Heterokaryosis clearly provides for additional adaptive flexibility (for example to changing environments) beyond that afforded by the more regulated conditions of dikaryosis or diploidy, and it has other implications. Different cells or nuclei within the mycelium may differ in ploidy or by having undergone mitotic recombination (see parasexual cycle, below). Genomic ‘conflict’ occurs where selection acts in opposition at different levels. For instance, at the organelle level, selection could favor a particular nuclear type, yet disfavor the resultant heterokaryon nuclear ratio at the level of the mycelium as a whole (James et al. 2008). Different nuclear ratios have been reported for conidia versus mycelium of the basidiomycete Heterobasidion parviporum, indicating that nuclei may compete to be included in these asexual propagules (James et al. 2008; Roper et al. 2011).
Conflict also arises because of the different degrees and modes of somatic transmission of the nuclear and mitochondrial genomes—the nuclei being relatively freely exchanged between the homokaryons whereas the mitochondria in the resultant dikaryon being contributed only from the ‘female’ parent (Anderson and Kohn 2007). More on these interesting points is discussed in the context of the genetic individual later in this chapter and in Chap. 5). For now, the important concluding point is that we see in heterokaryosis the ability of an organism to adjust the proportion of different sets of genes in response to environmental variation (e.g., available substrates). This is distinct from the formal mitotic-meiotic system of macroorganisms where the genotype (apart from somatic mutation) is continuous throughout the soma. The heterokaryotic fungus adapts genetically and physiologically literally as it grows. Successful heterokaryons, manifested by vegetative fusion and regulated nuclear exchange, as opposed to growth inhibition and cell lysis, are also evidence of nonself recognition systems in fungi, discussed later (Worrall 1997; Saupe 2000; Glass and Kaneko 2003). Finally, there are close parallels between this fungal system and cell/individual compatibility in colonial benthic invertebrates (e.g., Rosengarten and Nicotra 2011), a theme developed in Chap. 5.
Parasexuality Another distinctive attribute of the sexual process in fungi involves genetic recombination outside the usual sexual mechanisms. Some fungi, such as the opportunistically pathogenic yeast Candida albicans that were once thought to be asexual, have subsequently been shown to have a nonmeiotic parasexual cycle (Heitman 2006; Forche et al. 2008; Hickman et al. 2013). In the classic parasexual cycle there are four unrelated phases, each of which occurs relatively rarely (Pontecorvo 1946, 1956): (i) a heterokaryotic condition is established, as described above; (ii) diploidization (nuclear fusion) occurs giving a somatic, heterozygous, diploid nucleus; (iii) as growth ensues, the numbers of all nuclei increase by mitosis; in the diploid nuclei, mitotic crossing-over occurs between homologous chromosomes and occasional mitotic error can produce aneuploids; (iv) haploidization follows eventually as chromosomes are lost randomly in successive mitoses. Recombination results both from mitotic crossing-over as well as from the haploidization process. Parasexuality, in being a consequence of these uncoordinated, fortuitous events, is a process distinct from standardized sexual recombination.
It cannot replace conventional meiotic sex as a means for recombining genes, and in any one generation contributes insignificantly to variation (Caten 1987). The irregular karyotic variation, however, may be advantageous in regulating physiologically important genes in C. albicans; and the parasexual cycle, in bypassing the conventional sporulation cycle of this pathogen, may contribute to its ability to live in prolonged association with its hosts as a commensal (Forche et al. 2008). Considerably more information is needed on the extent and significance of parasexuality in nature. Despite its apparent rarity, the process provides yet one more means for genetic recombination, especially in certain supposedly asexual organisms, and it illustrates how mitosis can play a role in genetic variability (Schoustra et al. 2007).
In overview, it is evident that the fungi are a genetically versatile transitional group that spans the gamut in means of transmitting genetic variability. They exhibit some of the orderliness (meiotic mechanisms) of the macro organisms, together with haphazard variation mechanisms akin to those of the bacteria. Their idiosyncratic life cycles and strange nuclear processes and arrangements may reflect mechanisms to control access to the germline comparable to the evolutionary forces that led to historecognition systems in animals (Buss 1987) and are discussed in detail in Chap. 6.
Date added: 2025-06-15; views: 206;
