Carbon and Energy as Resources
Regardless of their complexity, nutrients ultimately are resolvable into the essential atomic elements and from a functional standpoint they have energetic, structural, electrochemical, mechanical, or catalytic roles. Of the 94 naturally occurring elements in the Periodic Table, all organisms consist of some 30, of which C, N, P assume particular importance (Sterner and Elser 2002). Why this particular nonrandom sampling from the Table and of the elements available on Earth has been made by natural selection is itself an interesting question, explored elsewhere (Frausto de Silva and Williams 1991; Sterner and Elser 2002).
Carbon is included as a major dimension in the categorization of life forms because, apart from water, molecules based on C are the most abundant in all organisms and it is unique in its properties, including a proclivity to form polymers. In organisms C tends to occur in reduced (hydrogenated or energy-rich) organic forms as opposed to environmental sources that are preponderantly gaseous or present as bicarbonates or carbonates (oxidized or energy-poor). The energy cycle is thus closely coupled specifically to the carbon cycle, both because of what the chemical states of carbon imply energetically and because light energy is converted to chemical energy for carbon fixation in photosynthesis.
All living organisms can be categorized into broad groupings based on their energy sources and the chemical form of carbon they obtain. Energy as ATP is probably the common currency of all living things, because it is required for almost every activity. Energy can be harvested in three ways: as light energy directly from the sun, or as chemical energy from inorganic compounds or organic compounds. In the great diversity of life, there are still only two basic mechanisms for generating ATP: The first, electron transport phosphorylation (oxidative phosphorylation or respiratory-chain phosphorylation), involves the flow of electrons ‘downhill’ from inorganic or organic donors with a relatively negative redox potential (higher energy) to those of a relatively positive (lower energy) potential, tied to the synthesis of ATP from ADP and inorganic phosphate.
This may occur in cyclic and noncyclic photophosphorylation (photo trophic organisms only) and in respiratory chains (most organisms). The second mechanism, substrate-level phosphorylation, is not associated with the process of electron transport, and occurs when organic substrates containing high-energy level phosphoryl bonds are degraded. For example, metabolism of some intermediates in glycolysis (Embden-Meyerhof Pathway), such as the catabolism of phosphoenolpyruvate to pyruvate, is associated with the transfer of the phosphate group to ADP to generate ATP.
A fairly detailed and inconsistently defined terminology applies to energy and carbon dynamics. Nevertheless, it can be a useful guide provided that its limitations are recognized. Definitions appearing in ecology and biology texts are more general than those used by most microbiologists. Bacteriologists in particular use the term ‘substrate’ to refer to the nutrient component of a resource. Substrates used in laboratory culture are often well defined chemically and their physiological function is usually known. Accordingly, an organic or inorganic chemical is referred to as a carbon source or an energy source or a nitrogen source, and so forth. Depending on the particular microbe, specific components might be O2 or NO3- (electron acceptors), NH4+ (nitrogen source), H2 (inorganic electron donor), or glucose or acetate (which are both organic carbon and energy/electron donor sources).
Another source of confusion is that the terms ‘autotroph’ and ‘heterotroph’ have been used variously across the biological disciplines to refer either to the energy source, or to the carbon source, or both (that is, unspecified). This may be because a large group of organisms, the animals (not to mention most microbes), use organic carbon both as a carbon source and as an electron donor (energy source). The scheme set out below and summarized in Table 3.2 follows largely that within bacteriology and is used here because it is both specific and universally applicable.
With respect to energy, biologically usable energy can be in the form of photons or chemical bonds. Organisms deriving their energy from light, which induces a flow of electrons for photophosphorylation, are phototrophs; those deriving electrons from a chemical energy source are chemotrophs. In either instance, the electron donor may be an inorganic or an organic substance. Lithotrophs use electrons from inorganic sources such as H2O, H2S, H2, Fe+2, S0, or NH4+. Organotrophs use organic substrates such as microbial, plant, and animal biomass that may be living or dead.
With respect to carbon source, some organisms can fix CO2 as their sole carbon source, in which case they are autotrophs. Organisms using mainly organic compounds rather than CO2 to supply cell carbon are heterotrophs. (As noted above, authors differ in their use of these terms; occasionally, for example, heterotroph and organotroph are used synonymously.) The options resulting from the energy/carbon source permutations form the basic categories for the functional categorization of creatures. Technically, and leaving aside for now various complications, any organism can thus be described by a 3-part prefix designating energy source/electron donor/carbon source (Table 3.2).
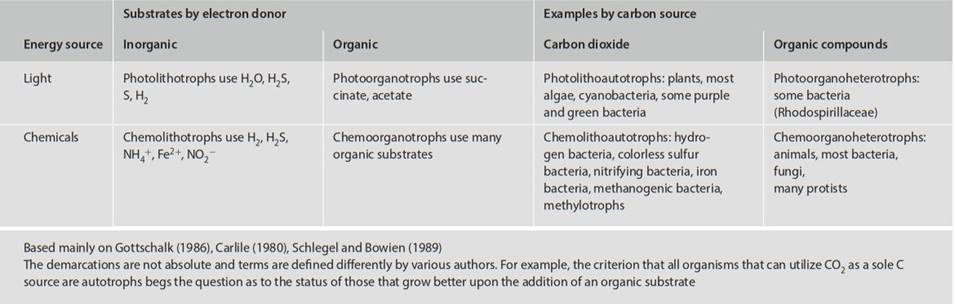
Table 3.2. Classification of organisms, with representative examples, based on energy source, electron donor, and carbon source
Plants are photolithoautotrophs and bears are chemoorganoheterotrophs. This terminology is cumbersome, so it is frequently abbreviated. Because most photolithotrophs also use inorganic carbon, they are usually simply called photoautotrophs. Likewise, most chemoorganotrophs use organic substrates both as a source of carbon and electrons and so are simply called chemoheterotrophs. As described later, certain nonphotosynthetic bacteria use light as a proton pump and in photophosphorylation, so phototrophy is not restricted to the process of photosynthesis alone; also, some organisms known as mixotrophs, discussed later, have combined the advantages of photoautotrophy and chemoheterotrophy.
Two observations on the information in Table 3.2 are particularly noteworthy. First, the apparent wide spectrum in patterns of energy and carbon source is really a result of metabolic diversity, and ultimately niche diversity, of the prokaryotes. Second, the resource patterns cross size boundaries. What biological properties belong exclusively to all members of a class, and what are the ecological implications? Is there a fundamental reason why the ability to form complex, multicellular macroorganisms is restricted to the photoautotrophs and the chemoheterotrophs? Why are there apparently no eukaryotic chemolithotrophs, either small or large?
A reflection of nutrient relationships is the well-known trophic structure of ecosystems (Bascompte 2009) based on food chains where a particular species, or more commonly group of species, occupies a trophic level. Organisms obtaining their food by the equivalent number of steps are by definition on the same trophic level. As historically depicted, the base or start of grazer chains (level 1) is formed by primary producers of organic matter, until recently assumed to be exclusively phototrophs that use sunlight as an energy source and consume inorganic nutrients and fix C autotrophically. Producers include plants, algae, photosynthetic bacteria and, remarkably, in some habitats (see below), chemosynthetic bacteria. All successive trophic tiers ordered basically by ‘who eats whom’ consist of consumer species, for example, herbivores (level 2) → first carnivores (level 3) .... → top carnivores (level n), i.e., they are energetic chemotrophs that use an organic source of carbon. The parallel in marine ecosystems is classically taken to be phytoplankton (collective term for the photoautotrophic microorganisms) and heterotrophic zooplankton grazers and their larger predators.
In contrast to grazer chains, detritus chains are based on dead organic matter, which supplies saprophytic microorganisms and detritivores that in turn are grazed by predators. (As discussed in the following sections, microorganisms and the lifestyle known as mixotrophy pose several complications to the conventional depiction.) The distinction between grazer and detritus chains is not as clear as it may seem and there is a close interaction between them. Trophic structure is really a functional, not a taxonomic, classification because many species (bears and raccoons are good examples) operate on more than one level. Chains interconnect into webs. Extensive destruction, as in massive deforestation (Laurance et al. 2006), or even the loss of selected species (Anderson et al. 2011; Estes et al. 2011), especially if they are critical or so-called keystone species (Paine 1969, 1995), can disrupt community dynamics across multiple trophic levels.
Food chains are short, generally 3-4 links measured vertically from the base to the top, though the webs of which they are a part vary substantially in their complexity (Post 2002; Brown et al. 2004; Pascual and Dunne 2006; Bascompte 2009). Of various hypotheses to explain the limitation on links, the most generally accepted ultimate constraint is attributed to energy loss within and between stages, coupled with energy requirements of animals at the top (an observation made famous by Hutchinson in 1959). Only about 1% of the light energy intercepted by phototrophs is converted to usable energy and of that only about 10% on average is passed through each step in the sequence. This is another area of complexity and debate, since the 10% level of efficiency is generally an educated guess. Transformation from level 2 to 3 has not been measured because what constitutes level 2 is often unclear and level 3 and above cannot be unambiguously defined in any real ecosystem. As one progresses up through a trophic sequence, the amount of usable energy entrapped at each level (kilocalories/square meter/year), the number of individuals (counts/square meter), and the amount of biomass (grams/square meter) accordingly usually decrease (energy does so invariably).
Trophic structure can be used to define particular communities or ecosystems, such as a lake or a forest, and can be depicted graphically as a pyramid based on energy or numbers (or biomass) of organisms at each level. The pyramidal shape for biomass or numbers is a measure of the standing crop of organisms at a point in time and reflects the fact that each species at level n + 1 sees only that portion of the resource at level n available to it. The shape for energy is more meaningful functionally than biomass or numbers as it is a measure of rate or flow of food production through the whole chain, not just the amount fixed at the level below (Odum and Barrett 2005).
The mechanistic basis of the energetic hypothesis for the pyramids is interesting, especially in an evolutionary context. The glycolytic reaction used to generate ATP is one of the oldest biochemical processes and is energetically very inefficient (2 molecules of ATP are produced per molecule of glucose catabolized). This energetic constraint means that the earliest heterotrophs arguably lived only as consumers of the chemo- and photoautotrophs, rather than of other heterotrophs. In other words, for some geological time the trophic chain would have consisted of a single link, with a large base of photoautotrophs or chemolithoautotrophs supporting a relatively small number of heterotrophs. It was the evolution of oxidative respiration, energetically an almost 20-fold improvement over glycolysis (36 molecules of ATP per molecule of glucose), that made successive tiers feasible. A possible evolutionary sequence that emphasizes the early role of microorganisms and relates changing food relationships to selection for increase in size is developed in Chap. 4.
Date added: 2025-06-15; views: 238;
