Energy and Carbon Sources: Key Drivers of Microbial and Species Distribution in Ecosystems
The sources of energy and carbon also set broad limits on the distribution of living things. Phototrophic micro- and macroorganisms illustrate this point well, being distributed with respect to light gradients (described later). The distribution of many species of lithotrophic microbes can be said to mirror environmental geochemistry: they reside in quantity only where their requisite inorganic electron donors reside. For example, Sulfolobus acidocaldarius lives primarily in sulfur-rich geothermal domains such as hot springs (Brock et al. 1972).
The organism is a thermophilic (temperature range 60-85 °C), aerobic, obligate acidophile (pH range 1-5). A member of the Archaea, it can thrive under hot, acid conditions in part because of an unusual cellular membrane, which is an exception to the ubiquitous phospholipid bilayer type. In these high-temperature habitats, H2S oxidizes spontaneously to elemental sulfur (S), which is in turn oxidized to H2SO4 by Sulfolobus, further acidifying the environment. The bacteria adhere to crystalline S thereby acquiring lithotrophically the few atoms going into solution that they need as an electron source (Fig. 3.3; Brock et al. 1972).
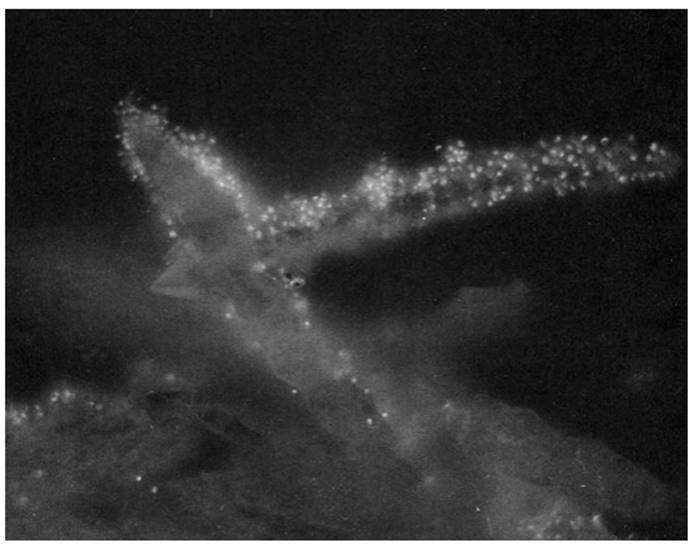
Fig. 3.3. A member of the Archaea, the sulfur-oxidizing Sulfolobus acidocaldarius attached to crystals of elemental sulfur. The archaeal cells appear as bright spots because they have been stained with the fluorescent dye acridine orange. Photo courtesy of T.D. Brock, University of Wisconsin-Madison
This, incidentally, is an excellent example of the importance of scale in ecosystems and of the unique ability of microbes, especially bacteria, to finely partition a resource: One microscopic S crystal, measuring on the order of cubic micrometers, can provide an energy source for many thousands of bacterial cells, whereas an entire plant or animal may be only one of many in the diet of a herbivore or carnivore. Sulfolobus also illustrates how specialization tends to impose limitations (e.g., a restricted range of resources) as well as opportunities (efficient harvesting mechanisms)!
The distribution of certain heterotrophs can also be strictly limited by that of their energy and carbon sources. Several examples are developed later (see section, Generalists and Specialists). For instance, the giant panda eats only bamboo and hence cannot exist in otherwise favorable regions of the world lacking its specific food source. Obligate parasites are restricted by the spatial and temporal availability of their hosts. Heteroecious parasites are those that need two or more hosts to complete their complex life cycles (Chap. 6). The fungus causing black stem rust of wheat (Puccinia graminis tritici) requires both wheat (in fact in agroecosystems it requires specific, susceptible cultivars of wheat) and common barberry (Berberis vulgaris) or other species of wild native barberry or mahonia. In the case of the Schistosoma flatworms, there is an obligatory alternation of a sexual generation in humans (occasionally other mammals) and an asexual generation in particular snails.
Terrestrial and aquatic plants, protists, animals, and algae (living or dead), provide locally high reservoirs of organic carbon as well as other nutrients, and accordingly act like nutritive islands for colonization by heterotrophic bacteria and fungi. Similarly, the pelagic zone, while impoverished as a whole, contains microenvironments enriched in nutrients. High microbial activity is associated, for example, with the relatively large fecal pellets of zooplankton and with ‘marine snow' which consists of heterogeneous flocculent aggregates containing phytoplankton, detritus, bacteria, and fecal pellets embedded in mucus.
Bacterial species known as oligotrophs predominate in nutrient-depleted waters, whereas copiotrophs tend to dominate in marine snow1 (Azam and Malfatti 2007; Lauro et al. 2009). Since many of these microorganisms, especially the oligotrophs, cannot be cultured, their lifestyle properties as well as predicted niches and trophic relationships are being inferred from their genomes (de Vargas et al. 2015; Lima-Mendez et al. 2015).
Unlike mobile animals and chemotrophic microbes, obligate phototrophs cannot choose their energy diets, but are of course affected by the intensity, temporal distribution, and spectral composition of light. Although plants vary in growth form, their energy gathering mechanisms for the common commodity, light, are essentially the same. Thus, they are unified by their need for a common energy source. This is in stark contrast to animals as a whole, which display extreme variation in diet and in anatomical features for harvesting their respective food sources. Such morphological differences among animals are analogous to the numerous architectures of plants. Once gathered and converted to precursor metabolites, however, the nutrient elements are handled biochemically and energy extracted (e.g., via glycolysis and the Krebs cycle) in much the same way by both groups of organisms, as alluded to in the Introduction.
Plants, algae, and phototrophic microbes are limited to areas where light occurs and hence do not grow in such habitats as caves, deep subterranean layers, or the intestines of animals. Remarkably, however, certain cyanobacteria and various green algae do grow inside porous rocks (endolithic phototrophs), where infiltrating water provides moisture as well as channels that facilitate access of light. In aquatic environments, the growth of attached macrophytes and benthic (bottom-dwelling) phototrophs, as well as phytoplankton, is restricted to the euphotic zone, which extends from the surface to a depth where photosynthetically active radiation is 1% of that at the surface. In general, this is sufficient for net photosynthesis to occur (i.e., photosynthesis above the compensation point, where photosynthesis equals respiration).
The depth of this zone varies with water clarity, but for coastal waters is typically about the uppermost 80 m; in oligotrophic waters, it may extend to about 200 m (Biller et al. 2015). In other words, within this zone energy is generally not the limitation to growth rate or yield, but rather nutrients frequently are limiting (Moore et al. 2013; Saito et al. 2014). The energy for phototrophs also arrives essentially in a continuous stream (albeit in quantum units), and does not entail hunting or trapping discrete packages separated in space and time, as the case for animals. Similarly, their energy supply is not subject to the laws governing population biology of prey in the sense that the energy source of chemoheterotrophs is influenced by the population dynamics of the plants and animals on which they live.
Within the euphotic zone, the distribution pattern of phototrophic organisms is related to light intensity and spectral quality that change with depth. Water absorbs proportionately more of the longer (red) than shorter (blue) wavelengths. Algae and cyanobacteria growing aerobically in the upper reaches of the water column further remove the red and some of the blue portions because these are the energy quanta preferentially absorbed by their chlorophylls and accessory pigments. Anaerobic, phototrophic purple and green sulfur bacteria commonly develop in a zone or on the muddy bottom (Fig. 3.4; Pfennig 1989) if the water is sufficiently clear to allow light penetration to depths that are anoxic, and in the presence of H2S, which is used as an electron donor. Thus, these organisms thrive under relatively specific, ecologically restricted conditions in stagnant water or on mud surfaces where they may form spectacular mats ranging in color from pink to purple-red or green.
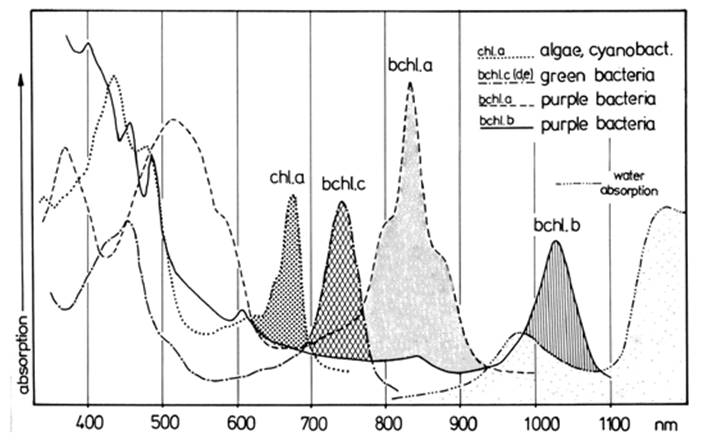
Fig. 3.4. Absorption spectra of representative phototrophic microorganisms as measured on intact cells. Note that light absorption by the algae and cyanobacteria as a whole is generally mid-spectrum (stippled area as shown for chl. a represented by the green alga Chlorella), while that of the green and purple sulfur bacteria is at longer wavelengths (bchl. c for Chlorobium; bchl. a for Chromatium, and bchl. b for Thiocapsa). Thus, light absorbed by the algae and cyanobacteria in shallow water or in floating mats does not appreciably reduce the wavelengths available for the sulfur bacteria. From Pfennig (1989). Reproduced by permission of Springer-Verlag, Heidelberg, ©1989
The phototrophic sulfur bacteria absorb primarily in the far red part of the spectrum and so can exploit wavelengths for anoxygenic photosynthesis not utilized by the algae. Their striking colors attributable to bacteriochlorophylls and carotenoid pigments are visible evidence of their resource-harvesting equipment. Adaptive divergence in pigment composition among photo- trophic phytoplankters allows for efficient partitioning of light energy and can favor coexistence (Stomp et al. 2004); alteration in pigment composition to maximize efficiency under prevailing conditions is also a well-known phenomenon (‘complementary chromatic adaptation’; Stowe et al. 2011).
Similarly, the role of preferential light absorption on the differential distribution of the cyanobacteria Prochlorococcus and Synechococcus in the photic zone is reviewed by Ting et al. (2002). The chemical equivalent of photic zonation is vertical zonation of prokaryote communities in marine sediments due to the successive depletion of electron acceptors for metabolism (Fenchel 2002). Photic stratification attributable to the effect of plant morphology on competition, as well as the ability to maintain positive energy income, is also evident in the depth zonation of aquatic plants in lakes and slow-flowing streams (Spence 1982).
In practical terms, knowing the energy and carbon sources of a particular organism is of great value to bacteriologists and mycologists in their attempts to isolate it in pure culture from nature. Isolation generally proceeds by enrichment, a process that provides favorable conditions for growth of the desired organism or, conversely, counter-selects against extraneous organisms. For example, use of a simple mineral salts medium with bicarbonate as a carbon source, together with appropriate light conditions, provide the basis for selecting photosynthetic bacteria. If incubation conditions are aerobic, cyanobacteria will be selected; if anaerobic (and in the presence of certain other growth factors and H2S as an electron donor), purple and green sulfur bacteria will be selected. Likewise, cellulose is commonly used as a carbon and energy source in isolating cellulolytic fungi.
Date added: 2025-06-15; views: 227;
