Optimal Digestion Theory: Bridging Rate vs. Efficiency Trade-offs in Animal and Microbial Resource Acquisition
Optimal digestion: trade-offs in rate versus efficiency. To be broadly applicable to resource acquisition, foraging should include optimal digestion theory because an important consideration is the dynamics of energy and nutrient extraction after the food source is acquired. This aspect concerns issues such as gut design, retention times, and absorption efficiencies in classic predators or analogous criteria for metabolic streamlining in plants and microorganisms. For example, broadly speaking, animals can maximize either digestion rate or digestion efficiency as discussed above in regard to search strategies. In principle, the longer the time that food is processed in the gut, the more nutrient or energy is extracted, up to the theoretical limit of what is available for processing.
Efficiency can be expressed as digestive efficiency (amount of usable resource extracted per unit food ingested) or in energetic terms as net energy obtained per unit of food ingested. Both generally increase over time up to a maximum. However, the rate of extraction can be visualized as rising to a maximum and then declining. A strategy of maximizing rate comes at the expense of digestion efficiency; organisms may maximize either rate or efficiency, or compromise at some intermediate level (Sibly and Calow 1986; Karasov and Martinez del Rio 2007) (Fig. 3.6).
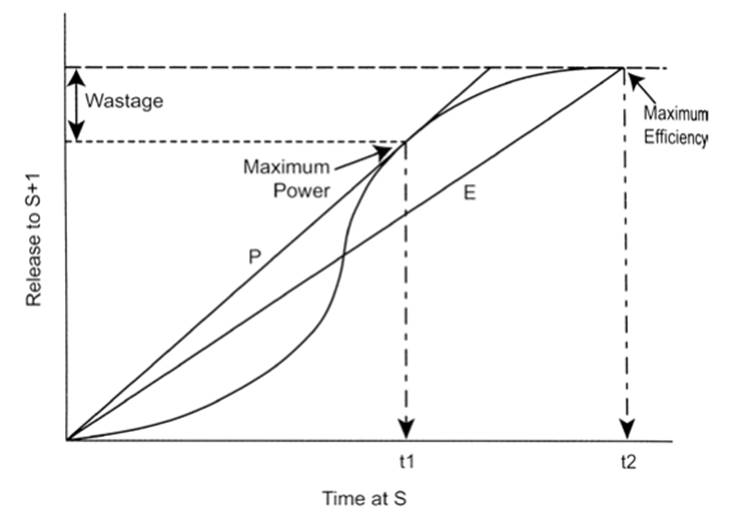
Fig. 3.6. Food processing in the animal gut showing the trade-off between maximum average rate versus maximum efficiency of digestion. Plot shows amount of nutrient released for post-digestive absorption as a function of time of digestion. The transfer function is shown by the sigmoid line. Upper dashed horizontal line shows maximum nutrient available. Nutrient transferred increases with time of processing in the gut with the greatest extent of transfer (efficiency) occurring at t2. The average rate of transfer for food held over time t2 is given by the line with slope E. However, the rate of transfer is maximized by holding food for a shorter period, t1, shown by the slope of line P. This occurs with a cost of ‘wastage’ in amount potentially transferred. From Physiological Ecology: How Animals Process Energy, Nutrients, and Toxins by W.H. Karasov and C. Martinez del Rio (2007). Reproduced by permission of Princeton University Press ©2007 via Copyright Clearance Center
Microbial behavior follows similar principles. There is considerable experimental and theoretical evidence that bacteria and other unicellular microorganisms such as yeasts are selected for either a rapid growth rate (cells per unit time) or high growth yield (total cells per unit substrate; Pfeiffer et al. 2001; Frank 2010). In parallel fashion, microbes appear generally able to harvest energy either quickly if wastefully (moles ATP per unit time), or efficiently (moles ATP per mole substrate). This leads to the situation where competitive outcome for a shared resource may be determined by rate of ATP production, whereas ultimate population size is determined not by rate of ATP synthesis but on ultimate ATP yield (Molenaar et al. 2009).
The inherent rate/yield trade-off is based on thermodynamics of chemical reactions where rate is negatively correlated with yield. Maximal rates of ATP production have been shown to result at intermediate yields (Pfeiffer et al. 2001; Molenaar et al. 2009). These dynamics are directly analogous to food processing in animals as illustrated in Fig. 3.6. For example, under aerobic conditions, at relatively low glucose concentrations and low growth rates, E. coli converts glucose to CO2 and water, generating ATP in high-yield fashion by respiration (38 mol ATP per mole glucose, which includes operation of both glycolysis and the citric acid cycle), as well as the precursor metabolites used in fueling reactions for biosynthesis. However, at high glucose concentrations and correspondingly high growth rates, also under aerobic conditions, cells partially redirect the glucose into energetically low- energy yielding (2 mol ATP net per mole glucose), fermentative pathways resulting in incomplete oxidation and the accumulation of by-products such as acetate (‘overflow metabolism’; Molenaar et al. 2009).
Why microorganisms switch from high to low-yield pathways has been explained in several ways (among them, saturation of the respiratory system; maintenance of redox balance; Molenaar et al. 2009; van Hoek and Merks 2012) and remains actively debated (Molenaar et al. 2009; Bachmann et al. 2013). The trade-off between rate and yield is a specific instance of a larger category of multi-objective decision-making models and as such has been called ‘an apparent Pareto front’ (Bachmann et al. 2013; for Pareto fronts and optimization, see, e.g., Shoval et al. 2012); this issue is closely related to r- and К-selection and will be discussed further in that context in Chap. 4.
As implied above, when resources become limiting, the relative advantage among competitors tends to change in favor of ability to extract energy. In a particularly interesting case, Brown et al. (1998) compared the growth dynamics of a parental strain (P) of baker’s yeast with an evolved (E) population derived from it that had undergone 450 generations of glucose-limited growth. In chemostat monoculture, the amount of residual glucose was 10-fold lower for E than P, indicative of the former’s enhanced scavenging ability. E out-competed P, attributed to multiple tandem mutations of high-affinity glucose transport genes that enabled it to achieve twofold greater yield biomass under substrate-limitation. In chemostat dual culture, the frequency of E continued to increase over time until P could no longer be detected. Interestingly, parallel experiments under nonlimiting conditions showed that this adaptation did not come at a cost in either yield or doubling time, i.e., there was no apparent trade-off. The advantage of E was attributable to its ability to transport limiting glucose 2-8 times faster than P and also an ability to produce more cells per mole glucose. In other words, it had both increased flux of limiting substrate into its cells, as well as a higher cell yield (for background theory and apparatus relevant to these experiments, see Gresham and Hong 2015).
Before we conclude this consideration of bacterial growth in culture, a perhaps obvious point nevertheless bears emphasis: Studying bacterial growth dynamics in culture can demonstrate capability but does not necessarily reflect behavior in nature. E. coli can grow very rapidly under favorable conditions and, like prokaryotes in general, is streamlined to do so. But this is still not as fast as theoretically possible. Why this is so is an old question (see, e.g., Koch 1988), still unanswered (Maitra and Dill 2015). The cell apparently trades-off growth speed and associated high costs of energy generation and biosynthesis for the ability to maintain seemingly wasteful ribosomal apparatus under slow-growth (maintenance) conditions. The reserve, however, ensures a fast start when transient, favorable times return. This strategy is entirely consistent with the feast-and-famine existence of E. coli in its enteric habitat (Koch 1971), and presumably it reflects as well the portion of the bacterium’s cryptic life history in the environment at large (Savageau 1983).
Finally, the rulebook for resource utilization by microorganisms differs in part from macroorganisms because for the latter starvation leads to death, whereas microbes under nutrient stress can shift easily to a dormant phase (Chap. 7). The evolutionary advantage of spore formation is clear in those habitats where drought or heat kills nonspore-formers. While this observation is true for the fungi and to a lesser extent for the bacteria (few of which form spores), it needs to be added that dormancy per se is not a productive venture for a microbe and can at best lead only to survival in place or distribution in space (Chap. 7).
However, ‘opting-out’ in suspended animation is time lost to growth and reproduction and hence to spreading one’s genes in the gene pool. Largely quiescent individuals would be selected out of the microbial milieu unless they counterbalanced prolonged dormant states with rapid growth, reproduction, and effective dispersal characteristics when transient favorable conditions arise. How different kinds of organisms handle the downshift to dormancy and the upshift to germination are correlates of r- and К-selection (Andrews and Harris 1986). These and other microbial strategies are discussed when the implications of size are considered (Chap. 4).
Integration of OFT and digestion. To summarize the foregoing, provided that the limitations of energy as a common currency are recognized, optimal foraging and digestion theory can serve as a useful conceptual framework for both microorganisms and macroorganisms. Nevertheless, it must be remembered that the architecture of an organism and its behavior are compromise responses to many selection pressures, of which energy acquisition and allocation is only one. The means by which very different organisms forage are not homologous but they are analogous (Table 3.3). For instance, animal ecologists refer to ‘foraging,’ to the ‘catchability of prey,’ to ‘handling time,’ to ‘feeding efficiency’ and so forth because the creatures that they study usually move about. In spinning a tensile web to trap its prey, the orb-weaving spider is behaving fundamentally like an aquatic filter-feeder that must expose a prey-capturing surface to water currents.
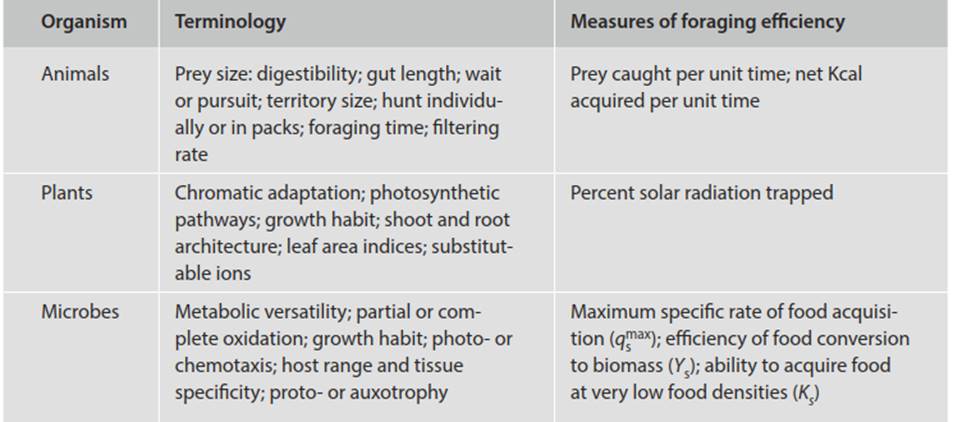
Table 3.3. Optimal foraging theory and optimal digestion theory broadly construed: some analogous components for animals, plants, and microbes
In essence, plant biologists deal with the same issue but in such terms as canopy architecture, photosynthetic pathways, leaf orientation, and optimal leaf area indices. Analogs in microbial ecology include such phenomena as enzyme kinetics and catabolite repression. For the bacterium or fungus that secretes extracellular enzymes to degrade organic matter there are chemical trade-offs—e.g., in producing the enzyme and in diffusional losses that can limit foraging distance. Pathologists and epidemiologists deal with host range, age or sex of suscept, or the specific tissues invaded.
Semantics and detail aside, the fundamental process of resource acquisition is universal, inevitably there will be trade-offs in resource capture, and an optimization approach can illuminate the analysis of adaptations. Alternative actions or strategies should be particularly analogous when compared within the group of organisms that is motile, or within the group that is collectively sedentary, regardless whether the organisms are microscopic or macroscopic.
Date added: 2025-06-15; views: 187;
